Posted inCardiology news Radiology
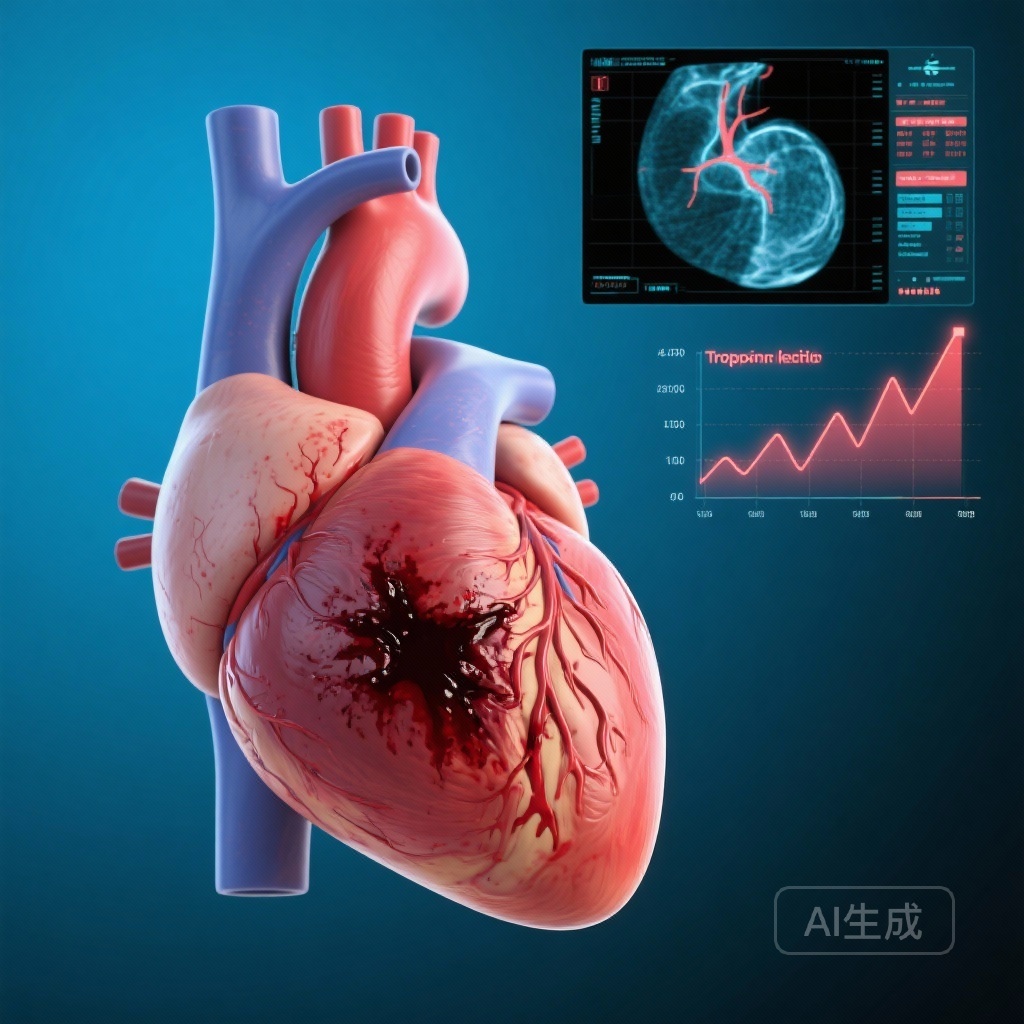
Post-PCI Troponin Kinetics Reveal Hemorrhagic Transformation as a Critical Driver of In-Hospital Mortality in STEMI
A multicenter study identifies high-sensitivity troponin I kinetics as a diagnostic marker for hemorrhagic myocardial infarction, linking this reperfusion injury to a nearly threefold increase in the risk of in-hospital mortality following primary percutaneous coronary intervention.