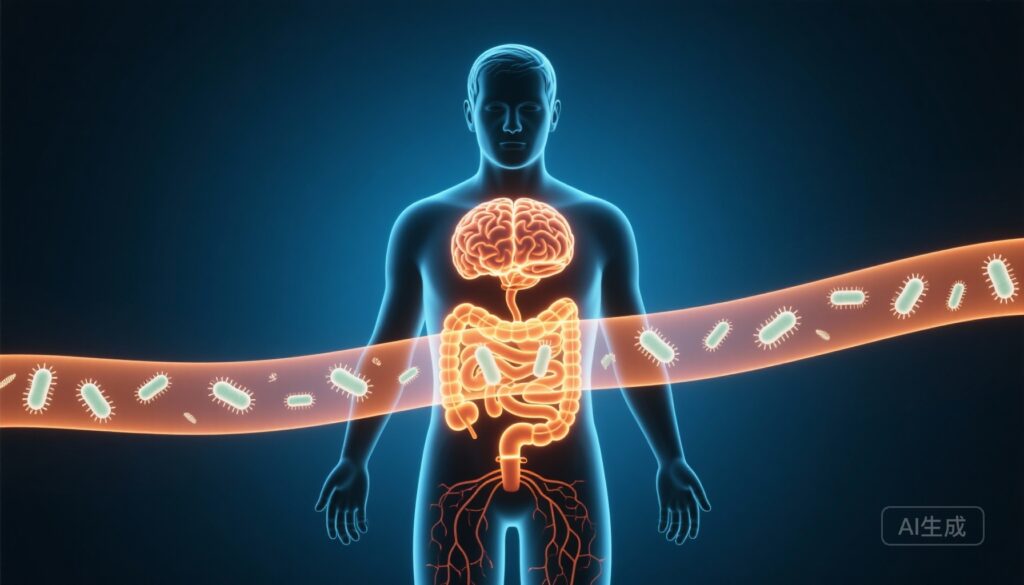
Highlights
– Large multi-cohort human study links gut microbial tryptophan metabolism to attention deficits in obesity, identifying 3‑hydroxyanthranilic acid (3‑HAA) as positively associated with attention and anthranilic acid (AA) as negatively associated.
– Shotgun metagenomics implicated Proteobacteria and microbial functions that convert AA into tryptophan as negatively associated with attention; bariatric surgery partially reversed these features.
– Causality and mechanism were supported by faecal microbiota transplantation (FMT) experiments in mice and behavioral assays in Drosophila, with modulation of serotonergic and dopaminergic pathways and prefrontal cortex (PFC) metabolomic/transcriptomic signatures.
Background
Cognitive impairments including deficits in attention are increasingly recognized co-morbidities of obesity. The gut microbiota influences host metabolism, immune function and neural systems via microbial metabolites that act locally and systemically. Tryptophan catabolism—through serotonergic and kynurenine pathways—produces metabolites that can modulate neurotransmission and neuroinflammation. Despite growing interest in gut–brain interactions, the specific microbial taxa, metabolic pathways and molecules that link obesity to attention remain poorly defined.
Study design
Castells-Nobau et al. conducted an integrated human–preclinical study (Gut. 2025) combining multi-omic analyses across three independent human cohorts and functional validation in animal models.
Key human components:
- Three cohorts with n=156, n=124 and n=804 participants were assessed for attention performance and profiled using faecal shotgun metagenomics and targeted plasma tryptophan metabolomics.
- Longitudinal changes after bariatric surgery were examined for effects on attention and microbiota composition.
Preclinical validation involved:
- Three separate FMT experiments transferring human donor microbiota into mice (diet-induced obesity models), with behavioural testing and PFC metabolomic and transcriptomic profiling.
- Mono-colonisation experiments in Drosophila melanogaster with Enterobacter cloacae and dietary manipulations to probe causal links and rescue studies using 3‑HAA supplementation.
Key findings
1) Clinical associations across cohorts
Obesity was reproducibly associated with reduced attention performance across cohorts. Integrative metagenomic analyses pointed to enrichment of Proteobacteria species and microbial functional modules that synthesize tryptophan from anthranilic acid (AA) in participants with poorer attention.
2) Plasma metabolomics and machine learning
Targeted profiling of plasma tryptophan metabolites combined with machine learning identified 3‑hydroxyanthranilic acid (3‑HAA) as positively associated with attention, particularly in individuals with obesity; by contrast, AA exhibited an inverse association. These relationships held after multivariable analyses, suggesting a robust signal across cohorts.
3) Bariatric surgery effects
Bariatric surgery, a weight-loss intervention known to alter the gut microbiome, improved attention scores and enriched microbial species previously linked to better attentional performance, consistent with a microbiome-mediated mechanism.
4) Mouse FMTs and PFC metabolomics
Diet-induced obesity (DIO) in mice and microbiota depletion both reduced concentrations of 3‑HAA and 5‑hydroxy-indole acetic acid (5‑HIAA, the main serotonin metabolite) in the prefrontal cortex (PFC). Transplantation of microbiota from high-attention human donors restored these metabolites in recipient mice.
Global metabolic profiling (>600 metabolites) of mouse PFCs after FMT identified the tryptophan and tyrosine pathways among the most significantly altered, with 3‑HAA notably enriched in mice receiving microbiota from high-attention donors.
5) Transcriptional changes
A second FMT experiment revealed concordant enrichment of tryptophan and tyrosine metabolic pathways at the transcriptional level in the PFC. Notably, genes involved in degradation pathways—Haao (3‑hydroxyanthranilic acid dioxygenase) and Aox4 (aldehyde oxidase 4), enzymes implicated in 3‑HAA and 5‑HIAA catabolism—were differentially regulated, supporting shifts in local metabolism that could alter neurotransmitter availability.
6) Behavioural transmissibility and neurotransmitter systems
In a third FMT study, attentional traits (deficits or preserved performance) were transmissible from humans to mice along with modulation of serotonergic and dopaminergic signalling in the PFC, linking microbial communities to host neurotransmission and behaviour.
7) Drosophila experiments
Mono-colonisation of Drosophila with Enterobacter cloacae in the context of a high-fat diet induced attention deficit–like behaviours in flies. Supplementation with 3‑HAA mitigated these deficits, providing cross-species functional evidence that 3‑HAA can influence attention-related circuits.
Mechanistic interpretation
The data support a model in which specific gut microbes modulate host tryptophan metabolism, shifting the balance between AA and downstream metabolites such as 3‑HAA and serotonin catabolites (5‑HIAA), with downstream effects on central neurotransmitter systems—particularly dopaminergic and serotonergic pathways in the PFC—that regulate attention.
3‑HAA lies within the kynurenine branch of tryptophan catabolism. It has pleiotropic properties: in some contexts it can be neuroprotective via antioxidant and immunomodulatory actions; in others, downstream conversion to quinolinic acid can contribute to excitotoxicity. The study’s observation that higher 3‑HAA associates with better attention and that supplementation rescues attention-like deficits suggests a context-dependent beneficial role in obesity-related cognitive dysfunction, possibly mediated by modulation of dopaminergic signalling and serotonin turnover in the PFC.
Clinical implications
These findings identify potential therapeutic targets to improve attention in people with obesity:
- Microbiota-directed interventions: dietary modification, targeted probiotics/synbiotics, bacteriotherapy or FMT strategies to enrich microbial functions that favor beneficial tryptophan metabolites.
- Metabolite-directed approaches: pharmacologic or nutraceutical modulation of 3‑HAA levels—though safety, dose–response and long-term effects require rigorous evaluation given the complex biology of kynurenine metabolites.
- Weight-loss interventions: bariatric surgery and perhaps other weight-loss strategies that alter gut ecology may have cognitive as well as metabolic benefits.
For clinicians, these data emphasize that attentional deficits in obesity may have a modifiable biological substrate linked to the gut microbiome, suggesting attention screening and multidisciplinary interventions could be considered in affected patients.
Strengths
The study’s strengths include replication across three human cohorts, an integrative multi-omic approach, and convergent functional validation across three FMT experiments and a Drosophila model, which together strengthen causal inference.
Limitations and caveats
Important limitations temper immediate clinical translation:
- Observational human data cannot by themselves prove causality; although FMT and invertebrate rescue experiments support transmissibility, human interventional trials are needed.
- Complexity of tryptophan metabolism: 3‑HAA has context-dependent effects and can be converted to downstream neuroactive compounds. Altering one node in this pathway may produce unintended consequences (e.g., increased quinolinic acid) unless carefully controlled.
- Species differences: while mice and flies provide mechanistic insights, human brain metabolism, blood–brain barrier transport and behavioural constructs of attention are more complex.
- Microbiome heterogeneity: specific taxa and functions varied; translating findings into precise microbial therapeutics will require strain-level characterization and safety evaluation.
Future directions
Key next steps include:
- Randomized interventional trials that manipulate microbiota (dietary, probiotic or FMT) or administer controlled 3‑HAA formulations to test effects on attention and neural biomarkers in humans.
- Detailed pharmacology and toxicology of 3‑HAA and related kynurenines to establish safe dosing windows and to monitor downstream metabolites like quinolinic acid.
- Mechanistic studies to delineate how peripheral microbial metabolites access or signal to the PFC—via blood–brain barrier transport, vagal pathways, immune signalling or endocrine routes—and to define cell types and receptors mediating effects.
- Personalized approaches integrating host genotype, diet and microbiome features to predict responders to microbiota- or metabolite-based therapies.
Conclusion
Castells-Nobau et al. provide compelling multi-level evidence that gut microbial modulation of tryptophan metabolism—particularly the balance between anthranilic acid and 3‑hydroxyanthranilic acid—links obesity to attention deficits via effects on prefrontal serotonergic and dopaminergic systems. While translational promise is high, careful human interventional studies and mechanistic safety work will be required before microbiota- or metabolite-targeted therapies enter clinical practice.
Reference
Castells-Nobau A, Fumagalli A, Del Castillo-Izquierdo Á, et al. Gut microbial modulation of 3-hydroxyanthranilic acid and dopaminergic signalling influences attention in obesity. Gut. 2025 Oct 9:gutjnl-2025-336391. doi: 10.1136/gutjnl-2025-336391.
Funding and trial registration
See the original publication for full funding disclosures and statements about ethical approvals. No clinicaltrials.gov registration number is reported in the primary reference for observational cohorts; any interventional studies arising from these findings will require formal registration.