Highlights
- Introduction of a hybrid AutoScore-Survival machine learning model integrating random survival forest variable selection with Cox regression for lung transplant outcome prediction.
- Use of extensive United Network for Organ Sharing data comprising over 50,000 adult lung transplant recipients spanning nearly four decades ensures model robustness.
- Identification of nine key clinical predictors including recipient and donor demographics, procedural details, and perioperative status enhancing personalized risk stratification.
- Model demonstrates moderate discrimination but excellent calibration and consistent net clinical benefit, supporting shared decision-making and transplant management.
Background
Lung transplantation remains the definitive therapy for a spectrum of end-stage pulmonary diseases. However, long-term survival after lung transplant exhibits considerable variability due to complex recipient, donor, and procedural factors. Current risk stratification tools suffer from limited accuracy, lack of interpretability, and suboptimal clinical utility, thereby impeding personalized prognosis and decision-making. The emergence of machine learning offers opportunities to harness large transplant registries for improved predictive modeling, but concerns over ‘‘black box’’ algorithms have restricted clinical adoption. This context underscores the imperative to develop interpretable, accurate, and clinically relevant models to predict time-to-event outcomes including death or retransplantation after lung transplant, facilitating risk stratification at meaningful time points such as 1, 5, and 10 years post-transplant.
Methos
A prognostic study was conducted using data on a cohort from the UNOS-OPTN registry, which captures detailed information on recipients of a lung transplant from across the US. This report followed the Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis + Artificial Intelligence (TRIPOD+AI) reporting guidelines. This study was conducted using deidentified secondary data from the UNOS registry. The study protocol was reviewed by the institutional review board at University of Texas Southwestern Medical Center and was determined to be exempt from the requirement for informed consent because no direct patient or public involvement was included in the research.
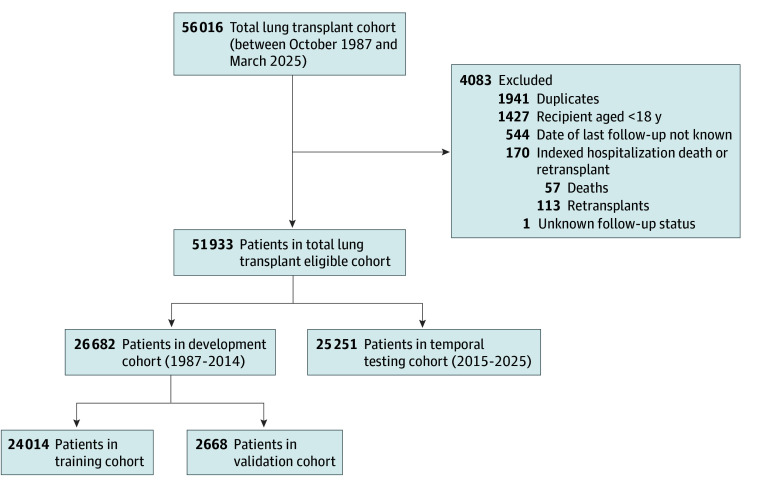
Figure 1. Patient Selection and Study Cohort Allocation.
Key Content
Chronological Development of Lung Transplant Outcome Prediction Models
Early outcome prediction models relied on conventional statistical approaches incorporating limited clinical variables, with variable performance and external validity. More recently, machine learning frameworks like random forests, neural networks, and gradient boosting have been explored in lung transplant cohorts to capture nonlinear relationships and complex interactions. Despite advancements in predictive accuracy, these models often lacked transparency, complicating bedside interpretability and clinical uptake. Hybrid approaches combining machine learning-driven variable selection with traditional regression-based scoring have emerged as promising strategies balancing performance and explainability.
Methodological Advances: AutoScore-Survival Framework
The referenced study leveraged the AutoScore-Survival framework, a novel hybrid pipeline that utilizes random survival forests for robust variable selection from high-dimensional data, followed by Cox proportional hazards regression to derive interpretable risk scores. This approach offers several advantages: data-driven selection reducing overfitting; survival-time modeling preserving temporal information; and transparent risk score calculation conducive to clinical use. Temporal data splitting (1987-2014 for development, 2015-2025 for testing) and additional internal validation ensured generalizability and optimal hyperparameter tuning.
Key Predictors of Lung Transplant Outcomes Identified
Nine predictors dominated the final model incorporating demographic, clinical, procedural, and laboratory parameters: length of hospital stay, recipient age, single versus double lung transplant, posttransplant ventilation support, prior cardiac surgery, serum creatinine at transplant, functional status, total bilirubin level, and donor age. These factors reflect pathophysiological impact (renal and hepatic function), surgical complexity, preoperative morbidity, and donor quality.
Model Performance and Clinical Utility
In the testing cohort (n=25,251), the model exhibited moderate discrimination (integrated AUC 0.61; Harrell C-index 0.64), with time-dependent AUCs of 0.61 at 1 year, 0.59 at 5 years, and improving to 0.72 at 10 years post-transplant, indicating increasing predictive accuracy over longer horizons. Calibration plots confirmed agreement between predicted and observed outcomes, substantiated by favorable Brier scores and observed-to-expected event ratios. Importantly, decision curve analysis demonstrated a consistent net benefit across clinically relevant threshold probabilities, supporting meaningful risk stratification to guide personalized management.
Figure 2. Kaplan-Meier Estimates of Survival by Risk Score.
Expert Commentary
This study represents a methodological and translational advance in lung transplant prognostication by synergizing machine learning’s variable selection capabilities with the interpretability of Cox regression. The use of a large, longitudinal, real-world dataset enhances external validity and applicability across diverse patient populations and eras. Although discrimination is moderate, this aligns with the inherent complexity and multifactorial determinants of lung transplant outcomes beyond available variables. The model’s strengths lie in good calibration, interpretability, and demonstration of clinical net benefit, critical for shared decision-making in transplant candidacy evaluation, post-transplant follow-up intensity, and counseling.
Despite these strengths, limitations include reliance on registry data with inherent missingness, potential unmeasured confounders (e.g., immunologic markers, adherence), and moderate discrimination that may not fully capture latent risk factors. Future research could integrate molecular, functional imaging, and comprehensive social determinants of health data to enhance predictive performance. Additionally, prospective validation in diverse transplant centers and evaluation of model integration into clinical workflows and patient outcomes are warranted.
Mechanistically, identified predictors such as renal and hepatic function markers reflect systemic organ reserve critical to withstand surgery and immunosuppression. Ventilation support and length of hospital stay provide surrogates for perioperative complications. Differentiating risk by single versus double lung transplant acknowledges procedural complexity and graft function variance.
Conclusion
The hybrid AutoScore-Survival model provides an interpretable, clinically meaningful tool for personalized risk stratification of lung transplant recipients. By accurately predicting time to death or retransplant at key milestones, it supports informed clinical decisions and patient counseling. Accessibility via a web-based calculator facilitates bedside implementation. Continuous refinement with emerging biomarkers and prospective validation will be essential to further improve prognostication, optimize resource allocation, and enhance transplant outcomes.
References
- Sharma G et al. Development and Validation of a Hybrid Machine Learning Model to Predict Lung Transplant Outcomes. JAMA Netw Open. 2025 Nov 3;8(11):e2545369. doi: 10.1001/jamanetworkopen.2025.45369 IF: 9.7 Q1 . PMID: 41288975 IF: 9.7 Q1 ; PMCID: PMC12648352 IF: 9.7 Q1 .
- Weill D, Benden C, Corris PA, et al. A consensus document for the selection of lung transplant candidates: 2014–an update from the Pulmonary Transplantation Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2015 Jan;34(1):1-15. doi:10.1016/j.healun.2014.06.014 IF: 6.0 Q1 .
- Palmer SM, Tevar AD. Optimizing recipient outcomes in lung transplantation. J Thorac Dis. 2016 Oct;8(Suppl 10):S827-S837. doi:10.21037/jtd.2016.07.65 IF: 1.9 Q3 .
- De Vries APJ, Vonk-Noordegraaf A, van Rees JB, et al. The use of machine learning in predicting survival after lung transplantation: current status and future perspectives. Transplant Rev (Orlando). 2020 Jan;34(1):100516. doi:10.1016/j.trre.2019.100516 IF: 2.5 Q2 .