Highlights
- EMG-guided Onabotulinum toxin A (50U) significantly reduced pain Visual Analogue Scale (VAS) scores at 3 months compared to placebo (p=0.008).
- Treatment led to sustained improvements in the Dermatology Life Quality Index (DLQI) at both 3-month and 6-month follow-ups.
- Significant enhancements were observed in sexual desire and sexual satisfaction according to the Female Sexual Function Index (FSFI).
- The study confirms that muscular dysfunction and hypertonicity are critical components of the pain profile in provoked vestibulodynia.
Background and Disease Burden
Provoked vestibulodynia (PVD) is the most common cause of localized dyspareunia in premenopausal women, affecting approximately 8% to 15% of the female population at some point in their lives. Characterized by severe, sharp pain upon contact with the vaginal vestibule—whether during sexual intercourse, tampon insertion, or gynecological examinations—PVD exerts a profound negative impact on psychological well-being, interpersonal relationships, and overall quality of life.
Despite its prevalence, the etiology of PVD remains complex and multifactorial, involving peripheral and central sensitization, inflammatory processes, and neurovascular changes. A significant subset of patients also exhibits abnormal pelvic floor muscle activity, specifically hypertonicity and impaired relaxation of the superficial perineal muscles, including the bulbospongiosus and ischiocavernosus muscles. Traditional management often involves a multimodal approach, including topical anesthetics, pelvic floor physical therapy, and cognitive-behavioral therapy. However, many patients remain refractory to these interventions. Onabotulinum toxin A (BoNT-A) has emerged as a potential therapeutic agent due to its ability to inhibit acetylcholine release at the neuromuscular junction and modulate nociceptive neurotransmitters, yet high-quality randomized controlled trials (RCTs) have been sparse until now.
Study Design and Methodology
In a rigorous randomized, double-blind, placebo-controlled clinical trial, Pelletier et al. sought to evaluate the efficacy of EMG-guided BoNT-A injections specifically for PVD. The study population consisted of 60 women diagnosed with provoked vestibulodynia associated with clinical evidence of muscular hypertonicity.
Participants were randomized to receive either 50 units of Onabotulinum toxin A (diluted in saline) or a saline placebo. A distinguishing feature of this trial was the use of Electromyography (EMG) guidance to ensure precise delivery of the toxin into the superficial perineal muscles. The injections were administered bilaterally (50U total, 25U per side) into the target musculature. The primary endpoint was the change in the pain Visual Analogue Scale (VAS) score from baseline to 3 months post-injection. Secondary endpoints included assessments of quality of life via the Dermatology Life Quality Index (DLQI) and sexual function through the Female Sexual Function Index (FSFI), with data collected at baseline, 3 months, and 6 months.
Key Findings: Pain Reduction and Functional Recovery
Primary Outcome: Pain Management
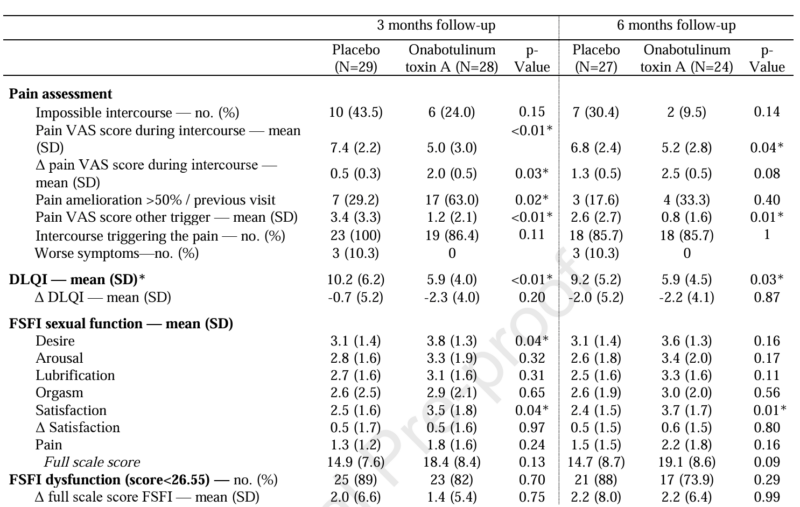
The trial achieved its primary endpoint with high statistical significance. At the three-month assessment, the BoNT-A group reported a mean pain VAS score of 5.04 ± 0.61, compared to 7.37 ± 0.45 in the placebo group (p=0.008). This reduction represents a clinically meaningful improvement in the daily lived experience of patients suffering from provoked pain. The results suggest that by targeting the hypertonic superficial perineal muscles, BoNT-A effectively breaks the cycle of pain-contraction-pain that characterizes PVD.
Secondary Outcome: Quality of Life
The impact of PVD extends beyond physical pain to dermatological and psychological distress. The study utilized the DLQI to capture these nuances. Patients in the BoNT-A group demonstrated significantly better DLQI scores at 3 months (p < 0.01) and maintained this improvement at the 6-month mark (p=0.03). This suggests that the benefits of a single BoNT-A injection session are not transient but persist through the medium-term recovery phase.
Secondary Outcome: Sexual Function and Satisfaction
Sexual dysfunction is a hallmark of PVD, often leading to secondary loss of desire and satisfaction. The FSFI scores revealed that the treated group experienced notable improvements in two critical domains: sexual desire and sexual satisfaction. Specifically, desire scores were significantly higher in the BoNT-A group (p=0.01), as were satisfaction scores (p=0.05). By reducing the physical barrier of pain, BoNT-A appears to facilitate a return to more positive sexual experiences, which is vital for the holistic recovery of the patient.
Mechanistic Insights and Clinical Commentary
The success of this trial provides compelling evidence for the “muscular hypothesis” of provoked vestibulodynia. While PVD was historically viewed primarily as a mucosal or neuropathic condition, these findings highlight that the superficial perineal muscles are often in a state of chronic hypertonicity or protective guarding. This muscle overactivity likely contributes to the compression of local nerve endings and the maintenance of peripheral sensitization.
The use of EMG guidance is a critical methodological strength of this study. Given the small size and specific location of the bulbospongiosus and ischiocavernosus muscles, blind injections carry a risk of inaccurate placement, which may explain the inconsistent results seen in earlier, non-guided BoNT-A studies. Precision targeting ensures that the neurotoxin acts directly on the motor endplates responsible for the localized hypertonicity.
From a clinical perspective, BoNT-A should be considered for patients who demonstrate clear signs of pelvic floor overactivity during physical examination (e.g., positive cotton-swab test combined with palpable muscle tension). While the study used a 50U dose, further research may be needed to determine if higher doses or repeated injection cycles offer additional benefits for more severe or chronic cases.
Expert Commentary
The results of the Pelletier et al. trial represent a significant step forward in the evidence-based management of PVD. By providing high-level evidence for BoNT-A, this study offers clinicians a potent tool for patients who have failed conservative therapies. However, it is essential to integrate these injections into a broader multidisciplinary framework. BoNT-A serves as a “window of opportunity”—by reducing muscle tension and pain for 3 to 6 months, it allows patients to engage more effectively in pelvic floor physical therapy and desensitization exercises, which are crucial for long-term success.
One limitation to consider is the relatively short follow-up period of 6 months. While the DLQI remained improved, the long-term durability of pain relief and the potential need for re-treatment remain areas for future investigation. Additionally, the study’s focus on superficial muscles suggests that deeper pelvic floor hypertonicity (levator ani) might require different therapeutic considerations.
Summary and Conclusion
In conclusion, EMG-guided Onabotulinum toxin A injections into the superficial perineal muscles offer a safe and effective treatment for women with provoked vestibulodynia. The trial demonstrated a significant reduction in pain scores and substantial improvements in quality of life and sexual function. These findings emphasize that muscular dysfunction is a core component of the PVD pain complex. Clinicians should consider EMG-guided BoNT-A as a viable intervention for patients presenting with hypertonicity-associated vestibulodynia, potentially transforming the standard of care for this challenging condition.
References
- Pelletier F, Sérézal IG, Puyraveau M, Leroux F, Verollet D, Aubin F, Amarenco G, Parratte B. EMG-guided Botulinum toxin injections in provoked vestibulodynia: a randomized double-blind placebo-controlled clinical trial. Am J Obstet Gynecol. 2025 Dec 17:S0002-9378(25)00934-2. doi: 10.1016/j.ajog.2025.12.037 IF: 8.4 Q1 .
- Bornstein J, Goldstein AT, Stockdale CK, et al. 2015 ISSVD, ISSWSH, and IPPS Consensus Terminology and Classification of Persistent Vulvar Pain and Vulvodynia. Obstet Gynecol. 2016;127(4):745-751.
- Diomede F, D’Aurora M, Gugliandolo A, et al. Botulinum Toxin Type A: From Clinical Applications to New Theories on Mechanisms of Action. Int J Mol Sci. 2021;22(21):11940.