Introduction
Alzheimer’s disease (AD) remains a major global health challenge, with no fully approved oral disease-modifying therapies available to alter its progression. Recently, blarcamesine (ANAVEX®2-73), a sigma-1 receptor (SIGMAR1) agonist, has been investigated for its potential to restore cellular homeostasis and slow cognitive decline in early AD. The ANAVEX2-73-AD-004 Phase IIb/III trial provided encouraging findings, but subsequent commentary has raised important concerns regarding the robustness and transparency of the reported data. This article synthesizes both the trial outcomes and critiques to offer a balanced perspective on blarcamesine’s role in early AD treatment.
Clinical Trial Overview and Findings
The ANAVEX2-73-AD-004 trial was a randomized, double-blind, placebo-controlled study across 52 centers in five countries, enrolling 508 participants diagnosed with early stage (Stage 3) Alzheimer’s disease. Participants received either blarcamesine at medium (30 mg) or high dose (50 mg), or placebo, administered orally once daily for 48 weeks. A subset transitioned into an open-label extension study (ATTENTION-AD), completed in June 2024.
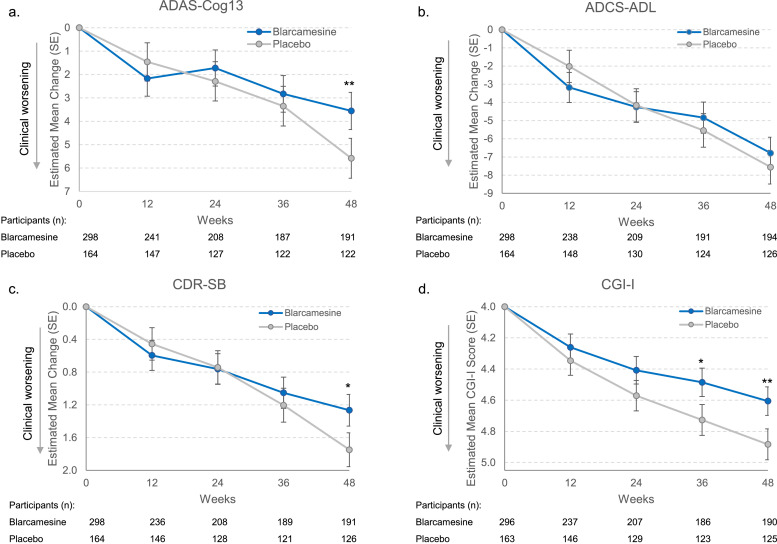
The trial’s co-primary outcomes focused on changes from baseline to 48 weeks in ADAS-Cog13 (a cognitive assessment) and ADCS-ADL (a functional abilities scale). Secondary outcomes included the Clinical Dementia Rating-Sum of Boxes (CDR-SB) and biomarker analyses: plasma Aβ42/40 ratio and MRI-measured global brain volume changes.
Results demonstrated statistically significant improvements favoring blarcamesine over placebo in ADAS-Cog13 (difference of -2.027 points, p=0.008) and CDR-SB (difference of -0.483 points, p=0.010). ADCS-ADL change did not reach significance. The plasma biomarker indicated a favorable increase in the Aβ42/40 ratio (p=0.048), and MRI assessments showed significantly less whole brain volume loss in the treatment groups (p=0.002).
Fig. Clinical efficacy endpoints estimated mean change from baseline, blarcamesine versus placebo, ITT population.
Safety profiles were comparable, with transient dizziness as the most common adverse event. Serious adverse events occurred in 16.7% of the blarcamesine group versus 10.1% in placebo, and mortality was not attributed to treatment.
Notably, subgroup analysis suggested subjects without the SIGMAR1 rs1800866 gene variant (wild-type) experienced greater cognitive benefits, underscoring a potential genetic influence on responsiveness.
Scientific Significance and Potential Impact
The trial results suggest blarcamesine may slow early cognitive decline by approximately 36.3% compared to placebo over 48 weeks. This effect magnitude is clinically relevant, particularly as a novel oral therapy targeting SIGMAR1, a receptor implicated in cellular stress response and neuroprotection.
If further validated, blarcamesine could offer an important alternative or complement to existing anti-amyloid therapies. The biomarker improvements reinforce the mechanistic plausibility of treatment effects, addressing both clinical symptoms and underlying pathology.
Critical Commentary and Concerns
Despite promising data, several concerns were raised regarding the trial’s analysis and reporting:
1. Tipping Point Analysis and Robustness: The sensitivity analysis used a tipping point method to assess data robustness to missing values. The reported tipping point indicated an observed difference of -1.973 was considered robust by the authors. However, critics argue that because this tipping point still favored blarcamesine over placebo, the data may not be as robust as claimed. Robustness should imply that the tipping point would have to be clinically implausible (e.g., showing a result worse than placebo) for significance to be lost. The current analysis did not meet this stricter standard, suggesting more caution is needed in interpreting results.
The upper graph, taken from the Lecanemab AdComm presentation in 2023 , and the lower graph, Figure 2A from the current report, both show red arrows I inserted marking the tipping points (with text boxes quoting the source). In each case, the length of the arrow corresponds to the reported tipping point.
2. Inconsistent Reporting: Discrepancies were noted between values reported at a scientific meeting in 2022 and subsequent publications in 2023 and 2025. The effect sizes for the primary cognitive endpoint ADAS-Cog13 varied (e.g., -1.85, -1.783, -2.027), raising questions about prespecified analyses versus exploratory data presentation. Since these analyses were filed with regulatory bodies under legal accuracy obligations, such inconsistencies call into question data management and transparency.
3. Protocol and Statistical Analysis Plan Availability: The trial protocol and statistical analysis plan, essential for evaluating study rigor, have not been made publicly available despite journal standards and repeated requests. Openness in these documents is critical to address concerns over analysis methodology and data interpretation.
Implications for Alzheimer’s Research Community
These critiques emphasize the need for transparency, consistent data reporting, and rigorous sensitivity analyses in AD clinical trials, particularly given the high stakes and prior controversies in the field.
Given that blarcamesine is under regulatory review (e.g., submitted to the EMA), addressing these issues promptly is crucial. Sharing protocol documents and clarifying analytical strategies will help bolster confidence among clinicians, researchers, and regulators.
Moreover, the ongoing emergence of oral small molecule SIGMAR1 activators provides a promising mechanistic avenue distinct from traditional amyloid-centric approaches, potentially widening therapeutic options for early Alzheimer’s patients.
Conclusion
The ANAVEX2-73-AD-004 trial delivers encouraging evidence that blarcamesine may slow cognitive decline in early Alzheimer’s disease, supported by biomarker improvements and a manageable safety profile. Nonetheless, the clinical research community must carefully scrutinize these findings in light of reported inconsistencies and demands for greater transparency.
Future studies with fully available protocols and rigorous analyses are essential to confirm blarcamesine’s efficacy and position it appropriately within the evolving Alzheimer’s therapeutic landscape.
Continued dialogue between sponsors, investigators, clinicians, and regulatory bodies will ensure that novel treatments for Alzheimer’s disease are developed and evaluated to the highest scientific and ethical standards, ultimately benefiting patients and families impacted by this devastating illness.
References:
Macfarlane S, Grimmer T, Teo K, O’Brien TJ, Woodward M, Grunfeld J, Mander A, Brodtmann A, Brew BJ, Morris P, Short C, Kurrle S, Lai R, Bharadwaj S, Drysdale P, Sturm J, Lewis SJG, Barton D, Kalafatis C, Sharif S, Perry R, Mannering N, MacSweeney JE, Pearson S, Evans C, Krishna V, Thompson A, Munisamy M, Bhatt N, Asher A, Connell S, Lynch J, Rutgers SM, Dautzenberg PL, Prins N, Oschmann P, Frölich L, Tacik P, Peters O, Wiltfang J, Henri-Bhargava A, Smith E, Pasternak S, Frank A, Chertkow H, Ingram J, Hsiung GR, Brittain R, Tartaglia C, Cohen S, Villa LM, Gordon E, Jubault T, Guizard N, Tucker A, Kaufmann WE, Jin K, Chezem WR, Missling CU, Sabbagh MN. Blarcamesine for the treatment of Early Alzheimer’s Disease: Results from the ANAVEX2-73-AD-004 Phase IIB/III trial. J Prev Alzheimers Dis. 2025 Jan;12(1):100016. doi: 10.1016/j.tjpad.2024.100016. Epub 2025 Jan 1. PMID: 39800452; PMCID: PMC12184016.
Brodkin J. Concerns about Anavex’s clinical trial of Blarcamesine. J Prev Alzheimers Dis. 2025 May;12(5):100137. doi: 10.1016/j.tjpad.2025.100137. Epub 2025 Apr 3. PMID: 40190003; PMCID: PMC12183999.