Highlight
Breakthrough Discovery
Researchers identified finerenone, an FDA-approved mineralocorticoid receptor antagonist, as a potent agent for restoring follicle growth in premature ovarian insufficiency (POI) by targeting the ovarian microenvironment rather than the follicles themselves.
Clinical Efficacy
Preliminary human trials demonstrated that oral finerenone (20 mg, twice weekly) successfully stimulated follicle maturation and the production of viable embryos in patients previously unresponsive to conventional gonadotropin stimulation.
Mechanistic Paradigm Shift
The study shifts the therapeutic focus from hormonal stimulation to the reduction of stromal fibrosis, proving that alleviating physical and biochemical constraints in the ovarian niche can reactivate dormant follicles.
Introduction: The Clinical Burden of Premature Ovarian Insufficiency
Premature ovarian insufficiency (POI) remains one of the most challenging diagnoses in reproductive medicine, affecting approximately 1% to 3% of women under the age of 40. Characterized by oligomenorrhea or amenorrhea, elevated follicle-stimulating hormone (FSH) levels, and low estrogen, POI often leads to permanent infertility. For many patients, the psychological and physiological impact is profound, yet clinical options remain severely limited.
Currently, there are no approved pharmacological interventions that reliably restore fertility in POI patients. Standard assisted reproductive technologies, such as In Vitro Fertilization (IVF), frequently fail because these patients lack antral follicles detectable by ultrasound. Even when residual primordial or primary follicles exist, they are often sequestered in a hostile ovarian environment that does not respond to exogenous gonadotropins. This lack of responsiveness has long been a brick wall in reproductive endocrinology, until now.
Study Design and High-Throughput Screening
In a landmark study published in the journal Science on February 5, 2026, a research team from the Li Ka Shing Faculty of Medicine at the University of Hong Kong (HKU) presented a novel strategy to overcome this barrier. Building on their previous research into the regulatory mechanisms of early follicle development, the team developed a screening platform to evaluate the potential of existing medications to stimulate folliculogenesis.
The researchers systematically screened a library of 1,297 FDA-approved compounds. Their goal was to identify drugs that could be repurposed to activate the growth of small, dormant follicles. Through this rigorous process, finerenone emerged as the leading candidate. Finerenone is traditionally known as a non-steroidal mineralocorticoid receptor antagonist used to treat chronic kidney disease associated with type 2 diabetes. However, its potent antifibrotic properties suggested a different utility in the context of ovarian biology.
Key Findings: From Mouse Models to Human Embryos
Preclinical Success in Murine Models
The researchers first tested finerenone in mouse models of ovarian insufficiency. They observed that the drug significantly promoted the development of ovarian follicles. Crucially, the study monitored the long-term safety of the intervention. There were no observed adverse effects on oocyte quality, early embryonic development, or the health and fertility of the resulting offspring. This established a robust safety profile for further translational steps.
Human Clinical Translation
Following the success in animal models, the team conducted preliminary clinical assessments. Women diagnosed with POI were administered oral finerenone at a dosage of 20 mg, twice weekly. The results were remarkable: the treatment facilitated the development of follicles in patients who had previously shown no response to hormonal stimulation. This resulted in the retrieval of mature oocytes and the successful creation of viable embryos. This represents a significant milestone, as it provides a tangible path to biological parenthood for women previously told their only option was egg donation.
Mechanistic Insight: Targeting the Ovarian Stroma
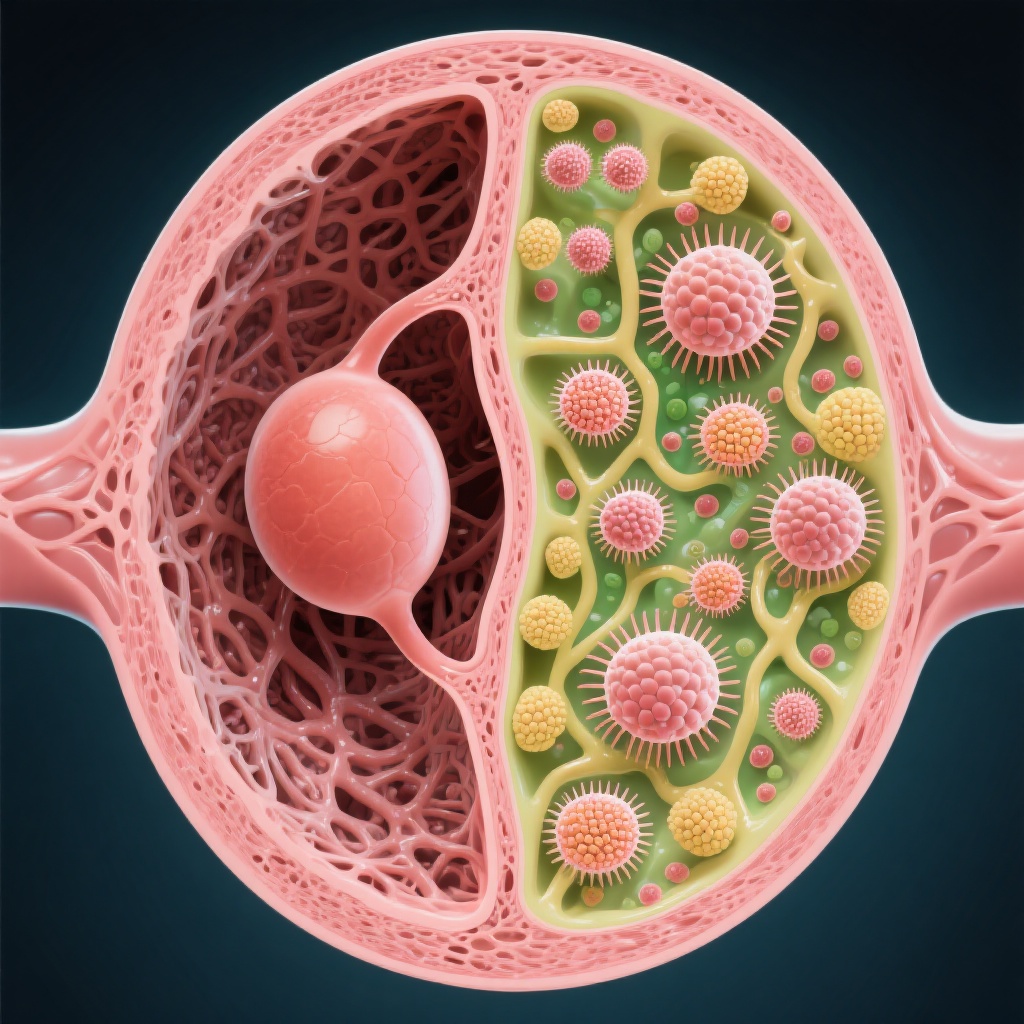
Historically, infertility treatments have focused on the follicles and oocytes themselves. This study, however, highlights the critical role of the ovarian “niche” or stroma. The researchers found that the primary barrier to follicle growth in POI is ovarian interstitial fibrosis—the excessive deposition of collagen in the ovarian tissue.
Breaking the Fibrotic Barrier
As the ovary ages or undergoes premature insufficiency, the stroma becomes increasingly fibrotic and stiff. This physical hardening creates a restrictive microenvironment that prevents small follicles from expanding and maturing. Finerenone acts by reducing this collagen deposition. By “softening” the ovarian environment, finerenone alleviates the mechanical and biochemical suppression of the follicles, creating a favorable microenvironment for their activation and subsequent growth.
Broader Antifibrotic Potential
To validate this mechanism, the researchers tested other FDA-approved antifibrotic drugs, including nintedanib (a tyrosine kinase inhibitor used for pulmonary fibrosis) and ruxolitinib (a JAK inhibitor). They found that these drugs, despite having different primary mechanisms of action, also effectively promoted follicle growth. This confirmed that interstitial fibrosis is a central pathological feature of POI and that targeting this state is a viable therapeutic strategy.
Expert Commentary and Perspectives
In a concurrent “Perspective” piece published in Science, Francesca E. Duncan of the Buck Institute for Research on Aging emphasized the significance of these findings. Duncan noted that by modulating the surrounding matrix—the “amorphous substance” enveloping the follicles—researchers have found a way to stimulate the remaining follicle pool in both aged mice and women with POI. This shift in focus from the germ cells to their supporting environment represents a paradigm shift in how we approach reproductive aging and disease.
While the results are highly promising, experts caution that larger, randomized controlled trials are necessary to fully establish the efficacy and long-term safety of finerenone for this specific indication. Furthermore, the optimal duration of treatment and the potential for combination therapies with traditional gonadotropins remain areas for future investigation.
Conclusion and Future Directions
The study from the University of Hong Kong provides a compelling evidence base for the use of antifibrotic drugs to treat POI-related infertility. By repurposing drugs like finerenone, clinicians may soon have a powerful tool to restore ovarian function. This research underscores the importance of the ovarian microenvironment and suggests that the future of reproductive medicine may lie not just in hormone replacement, but in stromal rejuvenation.
References
1. Science. (2026). Antifibrotic drug finerenone restores fertility in premature ovarian insufficiency. https://www.science.org/doi/10.1126/science.aee7270
2. Duncan, F. E., et al. (2026). Modulating the ovarian stroma to restore fertility. Science (Perspective).