Introduction: The Evolution of Vericiguat in Heart Failure Management
The management of heart failure with reduced ejection fraction (HFrEF) has undergone a revolutionary shift over the last decade. With the establishment of the four pillars of therapy—beta-blockers, ACE inhibitors/ARBs/ARNIs, mineralocorticoid receptor antagonists (MRAs), and SGLT2 inhibitors—clinicians have more tools than ever to improve survival. However, residual risk remains high, particularly following episodes of worsening heart failure. Vericiguat, a soluble guanylate cyclase (sGC) stimulator, was first validated in the VICTORIA trial, which focused on high-risk patients with a recent worsening event. The VICTOR trial was designed to extend these findings to a broader, more stable, and contemporary cohort of ambulatory HFrEF patients. The results, recently published in The Lancet and further analyzed in the Journal of the American College of Cardiology, provide a nuanced look at the drug’s efficacy and the changing landscape of heart failure monitoring.
The VICTOR Trial: Shifting Focus to Stable Outpatients
While the VICTORIA trial established vericiguat’s role in patients with a very recent hospitalization (within 3-6 months), the VICTOR trial (Vericiguat Global Study in Participants With Chronic Heart Failure) sought to determine if earlier initiation in a compensated, outpatient population would provide similar or greater benefit. This population represents the majority of HFrEF patients seen in routine clinical practice—those who are currently stable but remain at risk for future deterioration.
Study Design and Population Characteristics
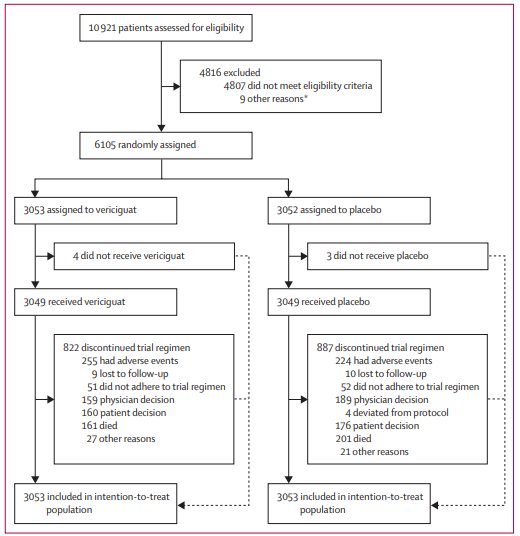
VICTOR was a phase 3, double-blind, placebo-controlled, randomized trial involving 482 sites across 36 countries. It enrolled 6,105 patients aged 18 or older with HFrEF (LVEF ≤40%). Crucially, the inclusion criteria required that patients had not experienced a heart failure hospitalization within the 6 months prior to randomization or used outpatient intravenous diuretics within the preceding 3 months.
Participants were randomized 1:1 to receive either oral vericiguat (titrated to a target dose of 10 mg) or a matching placebo. The median age was 68 years, with approximately 23.6% women and 76.4% men. A significant portion of the cohort (47.5%) had never been hospitalized for heart failure, making this a distinctly lower-risk population than that of the VICTORIA trial. Furthermore, the background guideline-directed medical therapy (GDMT) was robust, reflecting modern standards of care.
Primary and Secondary Outcomes: A Mixed Statistical Picture
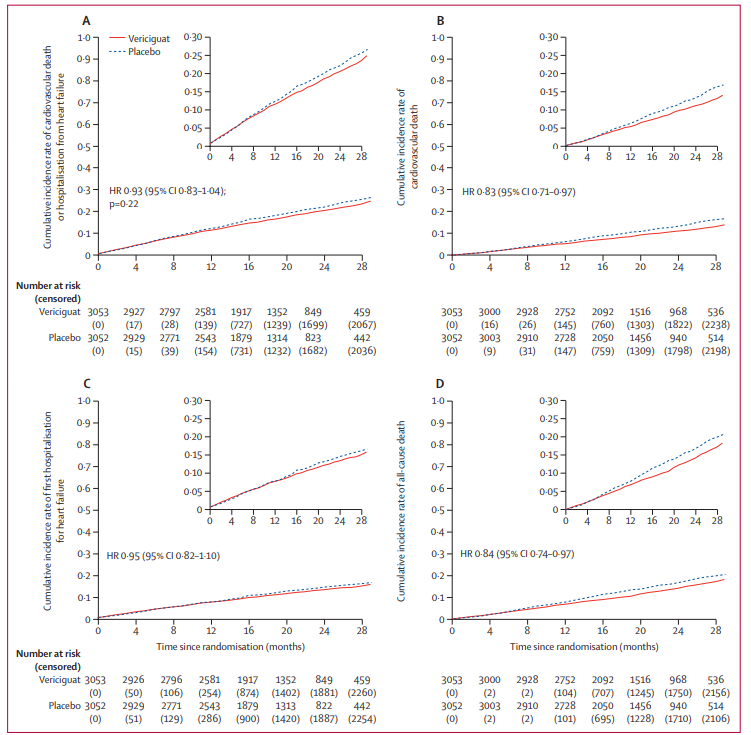
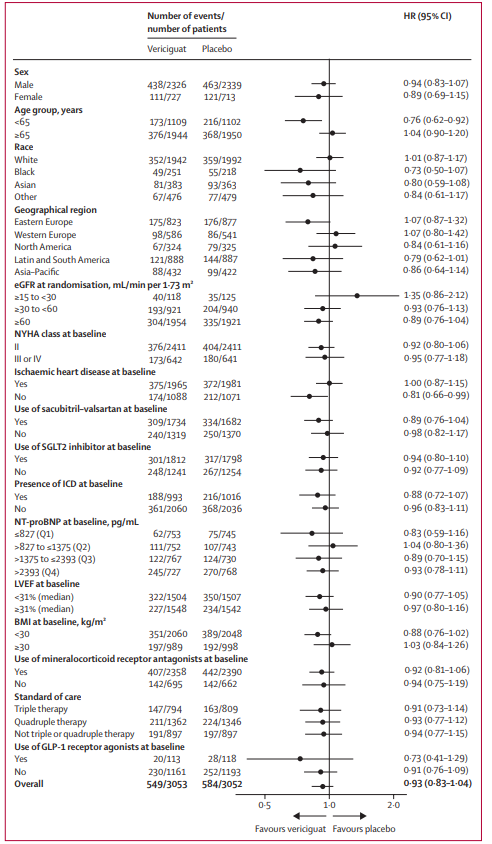
The primary composite endpoint of the VICTOR trial was the time to cardiovascular (CV) death or first hospitalization for heart failure (HHF). Over a median follow-up of 18.5 months, primary outcome events occurred in 18.0% of the vericiguat group and 19.1% of the placebo group. The resulting hazard ratio (HR) was 0.93 (95% CI: 0.83–1.04; p=0.22).
Because the primary endpoint did not reach statistical significance, all subsequent analyses of secondary and exploratory endpoints are considered nominal. However, these nominal results provide significant clinical insights that warrant closer inspection.
The Mortality Signal: Insights into Cardiovascular and All-Cause Death
Despite the neutral primary composite result, there was a notable divergence in mortality rates. Cardiovascular death occurred in 9.6% of the vericiguat group compared to 11.3% in the placebo group (HR: 0.83; 95% CI: 0.71–0.97). Similarly, all-cause death was lower in the vericiguat arm (12.3%) than in the placebo arm (14.4%), with an HR of 0.84 (95% CI: 0.74–0.97).
These findings suggest that while vericiguat may not have significantly altered the trajectory of the first hospitalization in this stable cohort, it may exert a protective effect on survival. This raises questions about whether the primary endpoint of time-to-first-event is the most sensitive metric for drugs targeting the sGC pathway in compensated patients.
Redefining Failure: The Significance of Outpatient Worsening
A critical secondary analysis of the VICTOR trial, published in JACC, addressed a potential limitation of the original study design: the underestimation of the heart failure burden in outpatients. In stable populations, many patients experience “worsening” that does not immediately result in hospitalization but requires outpatient intervention, such as oral diuretic intensification.
The Burden of Outpatient Events
The analysis revealed that outpatient worsening heart failure was actually more common than hospitalization as the first sign of clinical deterioration. Among the first worsening events recorded, 59.3% were outpatient oral diuretic intensifications, while only 35.4% were hospitalizations.
Importantly, the researchers found that outpatient oral diuretic initiation or intensification was a potent predictor of mortality, associated with a 69% increased risk of death (RR: 1.69; 95% CI: 1.47–1.94; P < 0.001). This confirms that even "minor" adjustments in the outpatient setting are markers of significant clinical progression.
Total Heart Failure Events: A More Comprehensive Patient Experience
When the analysis was expanded to include the entire experience of worsening—both inpatient and outpatient—the effect of vericiguat became more apparent. The composite of all-cause death and overall worsening heart failure occurred in 30.0% of the vericiguat group versus 32.9% of the placebo group (HR: 0.90; 95% CI: 0.82–0.98; P = 0.016). This exploratory analysis suggests that vericiguat may indeed provide a meaningful reduction in the total burden of heart failure when outpatient episodes are factored into the clinical equation.
Safety and Tolerability in a Compensated Cohort
Safety is a paramount concern when adding a fifth agent to a complex HFrEF regimen. In the VICTOR trial, serious adverse events were balanced between the groups (23.5% for vericiguat vs. 24.6% for placebo). The most common adverse event specifically associated with vericiguat was symptomatic hypotension, occurring in 11.3% of patients compared to 9.2% in the placebo group. This is consistent with the drug’s mechanism of action as a vasodilator but indicates that the medication is generally well-tolerated even in patients already receiving multiple GDMT agents.
Expert Commentary: Clinical Implications and Perspective
The results of the VICTOR trial present a paradox. By the strict definitions of clinical trial success, the trial was neutral. However, the consistent nominal reduction in mortality and the benefits seen when considering total worsening events suggest that vericiguat has biological activity in stable HFrEF.
Clinicians must weigh these results carefully. In the high-risk VICTORIA population, vericiguat is clearly indicated to reduce hospitalizations. In the VICTOR population, the benefit appears more focused on long-term survival and preventing the gradual slide into outpatient worsening. The trial also highlights the need for better monitoring of outpatient diuretic use as a surrogate for disease progression.
The study limitations include the hierarchical testing structure, which prevents the mortality findings from being definitive. Additionally, the high use of contemporary GDMT in the trial means that the incremental benefit of any new drug is increasingly difficult to prove statistically, even if it is clinically meaningful.
Conclusion: Future Directions for sGC Stimulators
The VICTOR trial underscores the complexity of treating HFrEF in the modern era. While the primary endpoint did not reach significance, the trial provides two major contributions to the field: first, it highlights that vericiguat is safe and potentially life-extending in a broad HFrEF population; and second, it demonstrates that outpatient worsening is a critical, yet often overlooked, component of the heart failure disease burden. Future guidelines may need to consider whether the cumulative evidence from VICTORIA and VICTOR supports a broader use of sGC stimulators across the spectrum of HFrEF stability.
Funding and Trial Registration
The VICTOR trial was funded by Merck Sharp & Dohme (a subsidiary of Merck) and Bayer. The trial is registered with ClinicalTrials.gov, number NCT05093933.
References
1. Butler J, McMullan CJ, Anstrom KJ, et al. Vericiguat in patients with chronic heart failure and reduced ejection fraction (VICTOR): a double-blind, placebo-controlled, randomised, phase 3 trial. Lancet. 2025 Sep 27;406(10510):1341-1350. doi: 10.1016/S0140-6736(25)01665-4.
2. Zannad F, Reddy YNV, Barash I, et al. Effect of Vericiguat on Total Heart Failure Events in Compensated Outpatients With HFrEF: Insights From VICTOR. J Am Coll Cardiol. 2025 Dec 16;86(24):2471-2491. doi: 10.1016/j.jacc.2025.08.051.