Posted inInternal Medicine news OB/GYN & Women’s Health
Antifibrotic Repurposing: How Finerenone Could Rejuvenate the Ovarian Niche and Restore Fertility in POI
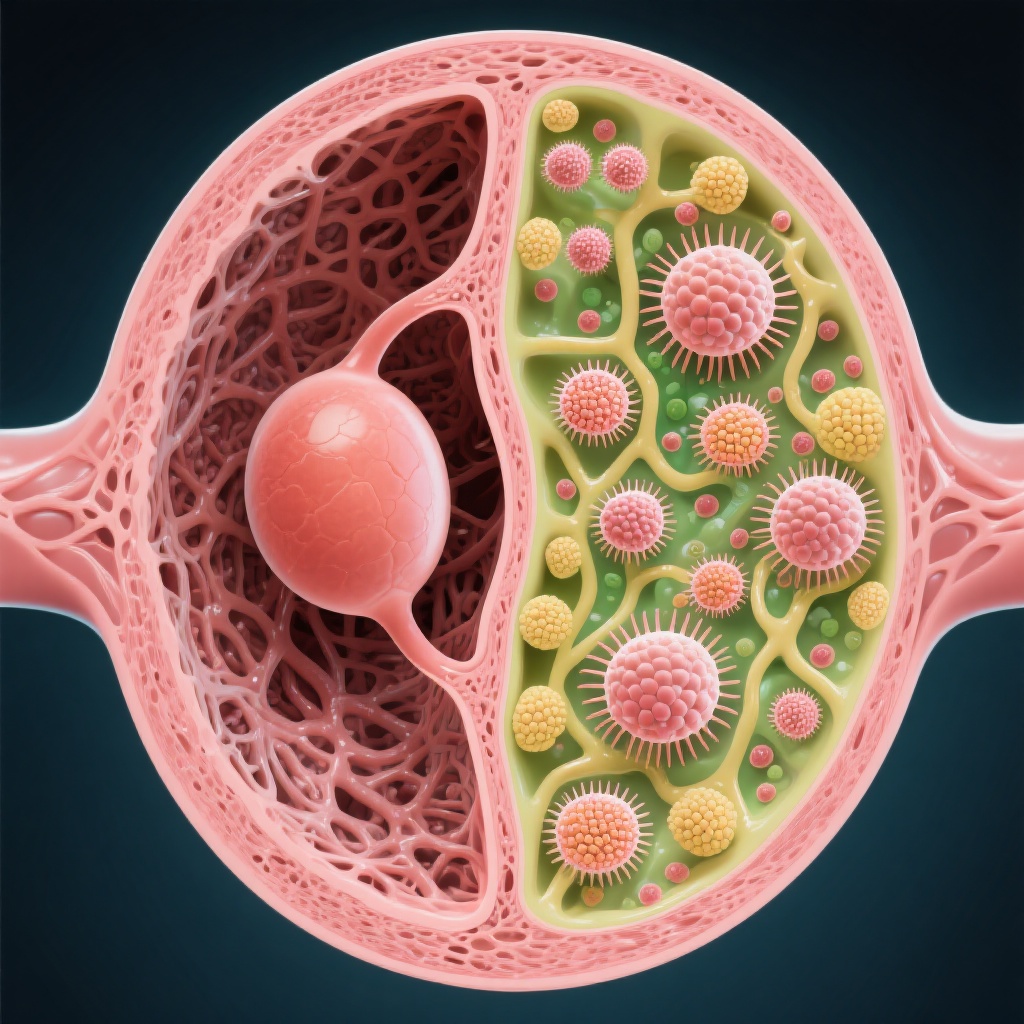
A breakthrough study in Science reveals that the antifibrotic drug finerenone restores fertility in premature ovarian insufficiency (POI) by reducing ovarian stromal fibrosis, enabling follicle development where hormone therapy previously failed.