Posted inCardiology news Radiology
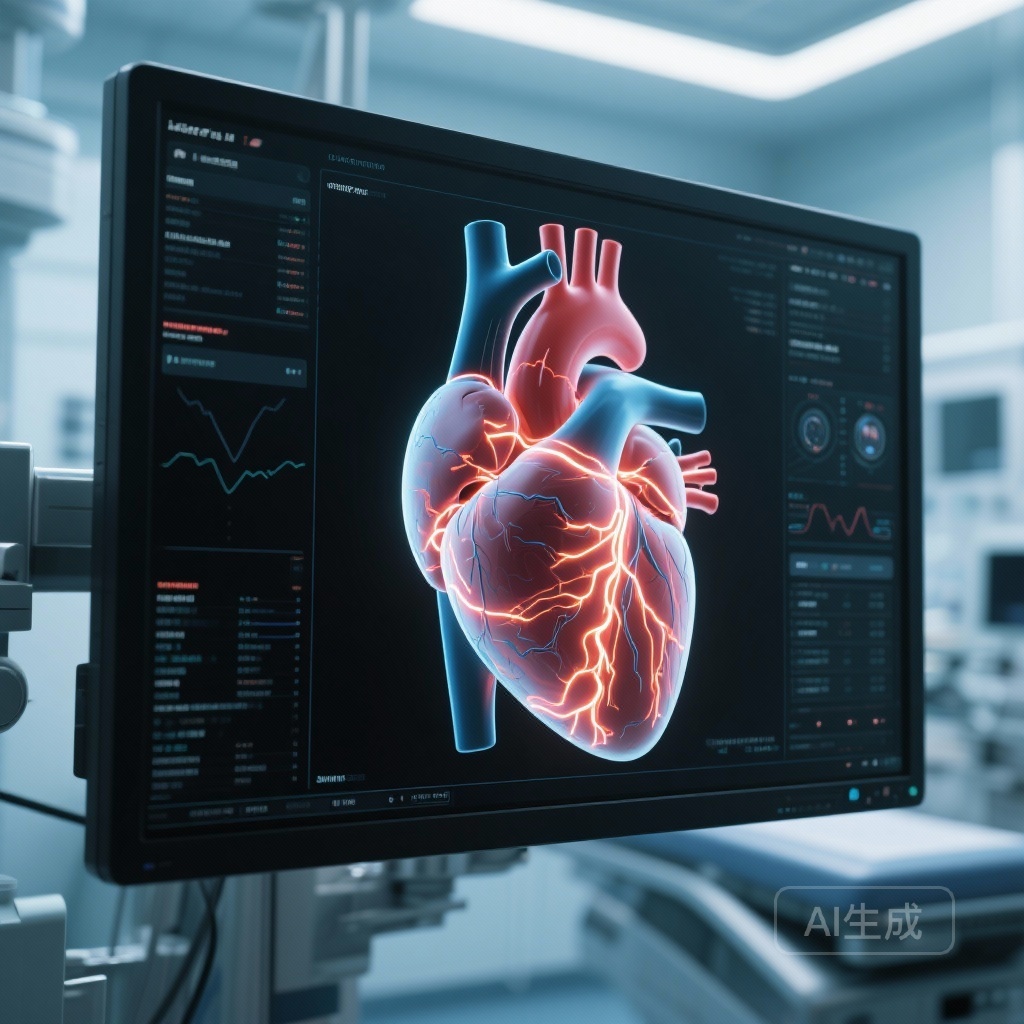
Precision Lead Placement: How 4D Phenomics Digital Heart Models are Revolutionizing Cardiac Resynchronization Therapy
The MAPIT-CRT trial demonstrates that using 4D cardiac MRI-generated digital heart models to guide lead placement significantly improves LVEF in heart failure patients compared to standard techniques, offering a safe and feasible precision medicine approach.