Highlights
- Initial RSV vaccine effectiveness (VE) against documented infection is high (82.5%), but wanes to approximately 59.4% over 18 months.
- Protection against severe outcomes remains more robust than against infection, with 71.9% VE against ICU admission maintained through the second season.
- Immunocompromised individuals experience significantly faster waning of protection, with VE against infection dropping to 39.7% by month 18.
- The study utilizes a target trial emulation design, providing high-quality real-world evidence (RWE) to complement initial phase 3 clinical trial data.
Background
Respiratory syncytial virus (RSV) has long been recognized as a major cause of morbidity and mortality in older adults, particularly those with underlying cardiopulmonary conditions or immunodeficiencies. Until recently, preventative options were limited to supportive care and infection control. The landmark approval of recombinant stabilized prefusion F protein vaccines marked a turning point in geriatric preventative medicine. While initial Phase 3 trials (such as RENOIR and AReSVi-006) demonstrated high efficacy within the first season, clinicians and policy experts have been concerned about the durability of this protection over subsequent seasons.
In the United States, the Advisory Committee on Immunization Practices (ACIP) initially recommended the vaccine for adults aged 60 and older using shared clinical decision-making, which later evolved into more specific age-based recommendations. However, the question of whether a single dose is sufficient for multi-season protection or if a seasonal booster approach—similar to influenza—is required remains a subject of intense debate. This review synthesizes new evidence from the Veterans Health Administration (VHA) regarding the long-term durability of RSV vaccines.
Key Content
Methodological Innovation: Target Trial Emulation
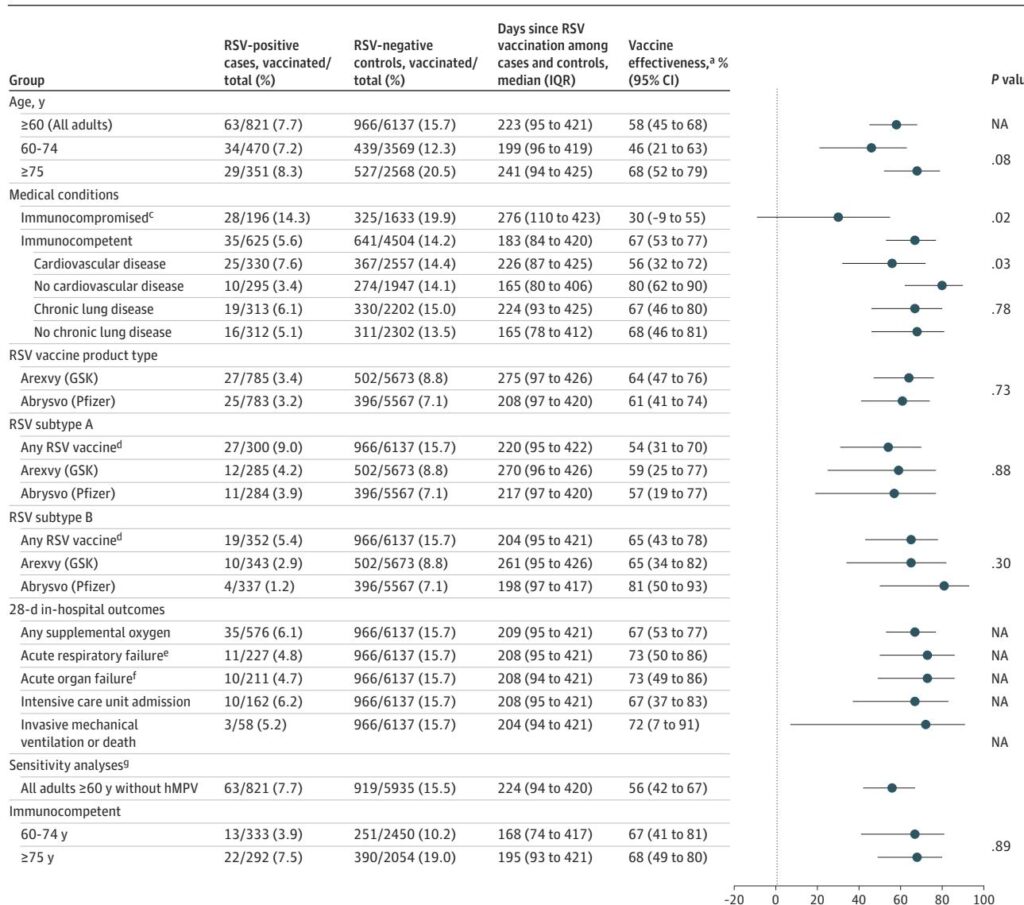
The study by Bajema et al. (2025) represents a sophisticated application of target trial emulation. By utilizing data from the VHA, the largest integrated healthcare system in the US, researchers matched 288,111 vaccinated veterans to over 1 million unique controls. This design is crucial for mitigating healthy-user bias, where individuals seeking vaccination are often more health-conscious or have better access to care than those who do not. The use of seven monthly, nested sequential trials allowed the investigators to account for the timing of vaccination relative to local RSV circulation patterns.
Durability of Protection Against Infection and Mild Illness
The primary outcome assessed was documented RSV infection. The findings indicate a clear temporal decay in immunologic protection:
- 0–1 Month: VE was estimated at 82.5% (95% CI, 77.5%-86.9%).
- Cumulative 18 Months: VE decreased to 59.4% (95% CI, 55.6%-63.5%).
This decline suggests that while the stabilized prefusion F protein induces a strong initial B-cell response, the neutralizing antibody titers or the memory T-cell response may not be sufficient to prevent breakthrough mucosal infections as time progresses and the virus evolves or the immune response contracts.
Protection Against Severe Outcomes and Healthcare Utilization
From a public health and clinical perspective, the prevention of severe disease—hospitalization and ICU admission—is the primary goal of vaccination in the elderly. The data showed a similar, albeit slightly more resilient, trend in these categories:
- Emergency Department/Urgent Care Visits: Waned from 84.9% to 60.5%.
- Hospitalizations: Waned from 88.9% to 57.3%.
- ICU Admissions: Remained relatively high, moving from 92.5% at the start to 71.9% at 18 months.
These results confirm that the vaccine remains highly effective at preventing the most catastrophic outcomes for at least two respiratory seasons, even as its ability to prevent outpatient infection diminishes.
Vulnerability in the Immunocompromised Subpopulation
A critical finding of the Bajema et al. study is the rapid waning observed in immunocompromised veterans. For this cohort, protection against documented infection fell from 75.2% in the first month to a mere 39.7% by 18 months. This highlights a significant clinical gap; these high-risk patients, who are most likely to suffer complications from RSV, lose nearly two-thirds of their vaccine-derived protection within two seasons. This divergence suggests that the “one-size-fits-all” single-dose strategy may be inadequate for the immunocompromised.
Expert Commentary
Biological Mechanisms of Waning
The waning effectiveness of RSV vaccines likely reflects the natural decay of serum neutralizing antibodies (nAbs). The stabilized prefusion F protein is highly immunogenic, but the respiratory tract’s reliance on localized IgA and systemic IgG means that as titers drop below a certain threshold, protection against upper respiratory infection is lost first. The relative preservation of protection against ICU admission suggests that cellular immunity (T-cell responses) may be more durable, providing a “safety net” that prevents viral dissemination to the lower respiratory tract and subsequent systemic inflammatory response.
Clinical Implications for VHA and Beyond
The VHA population is uniquely characterized by a high prevalence of comorbidities and a predominantly male, older demographic. While this makes the data highly applicable to older male veterans, caution must be exercised when generalizing these results to younger, healthier populations or to women. However, the sheer scale of this study provides some of the most robust RWE available to date.
For clinicians, the data supports the continued recommendation of the RSV vaccine for eligible older adults, given its strong performance in preventing ICU-level care. However, the data for the immunocompromised is a call to action. We must consider whether these patients require more frequent dosing or different vaccine formulations (e.g., higher doses or adjuvants) to maintain protective thresholds.
Policy and Future Directions
These findings will likely inform the ACIP’s future deliberations on the necessity of biennial or annual RSV boosters. If the 18-month VE for hospitalization continues to trend downward in future analyses, the case for a second dose prior to the third season will become compelling. Further research is needed to determine the safety and immunogenicity of subsequent doses and to identify precise biomarkers (correlates of protection) that can predict when an individual is no longer protected.
Conclusion
The investigation into RSV vaccine durability among US Veterans underscores a pivotal truth in vaccinology: initial efficacy does not guarantee permanent immunity. While the current RSV vaccines offer a formidable defense against severe respiratory failure and hospitalization over two seasons, the significant waning of protection against infection—especially in immunocompromised groups—highlights a need for tailored clinical strategies. As we move forward, the focus must shift from initial implementation to optimizing the long-term schedule to ensure that the most vulnerable members of the population remain shielded from the significant burden of RSV.
References
- Bajema KL, Bui DP, Yan L, et al. Durability of Respiratory Syncytial Virus Vaccine Effectiveness Among US Veterans. JAMA Intern Med. 2025;e256355. doi:10.1001/jamainternmed.2025.6355. PMID: 41284307.
- Papi A, Ison MG, Langley JM, et al. Respiratory Syncytial Virus Prefusion F Protein Vaccine in Older Adults. N Engl J Med. 2023;388(7):595-608. PMID: 36791533.
- Walsh EE, Marc GP, Zareba AM, et al. Efficacy and Safety of a Bivalent RSV Prefusion F Vaccine in Older Adults. N Engl J Med. 2023;388(16):1465-1477. PMID: 37018477.