Background
Gestational diabetes mellitus (GDM), affecting approximately 14.7% of pregnancies in the Western Pacific region, is characterized by glucose intolerance first recognized during pregnancy and carries significant risks of adverse maternal and neonatal outcomes, including macrosomia, cesarean delivery, and preeclampsia. Although diagnostic criteria mainly rely on oral glucose tolerance test (OGTT) glucose thresholds, this glucose-centric approach overlooks the considerable metabolic heterogeneity in GDM. Insulin secretion and resistance vary markedly among affected women and influence pregnancy outcomes, yet are not reflected by glucose measurements alone. C-peptide, produced equimolarly with insulin but less subject to hepatic clearance, serves as a reliable marker of endogenous insulin secretion. Incorporating dynamic C-peptide measurements during OGTT could thus provide deeper insight into GDM pathophysiology and aid risk stratification for personalized management.
Study Design
This nested case–control study drew upon the Xi’an Longitudinal Mother–Child Cohort, encompassing 1014 women diagnosed with GDM and 1014 matched normoglycemic controls delivering singleton live births between January 2017 and December 2018. All participants underwent a standardized 75-g 2-hour OGTT at 24–28 weeks gestation, with venous sampling for glucose, insulin, and C-peptide at fasting (0 h), 1 h, and 2 h. Latent class trajectory modeling (LCTM) was applied to C-peptide data to identify distinct dynamic secretion patterns. Associations between identified C-peptide trajectories and adverse fetal (e.g., large for gestational age [LGA], macrosomia) and maternal outcomes (e.g., preeclampsia) were analyzed using logistic regression, adjusting for relevant covariates including maternal age, prepregnancy BMI, family history of diabetes, and obstetric history.
Key Findings
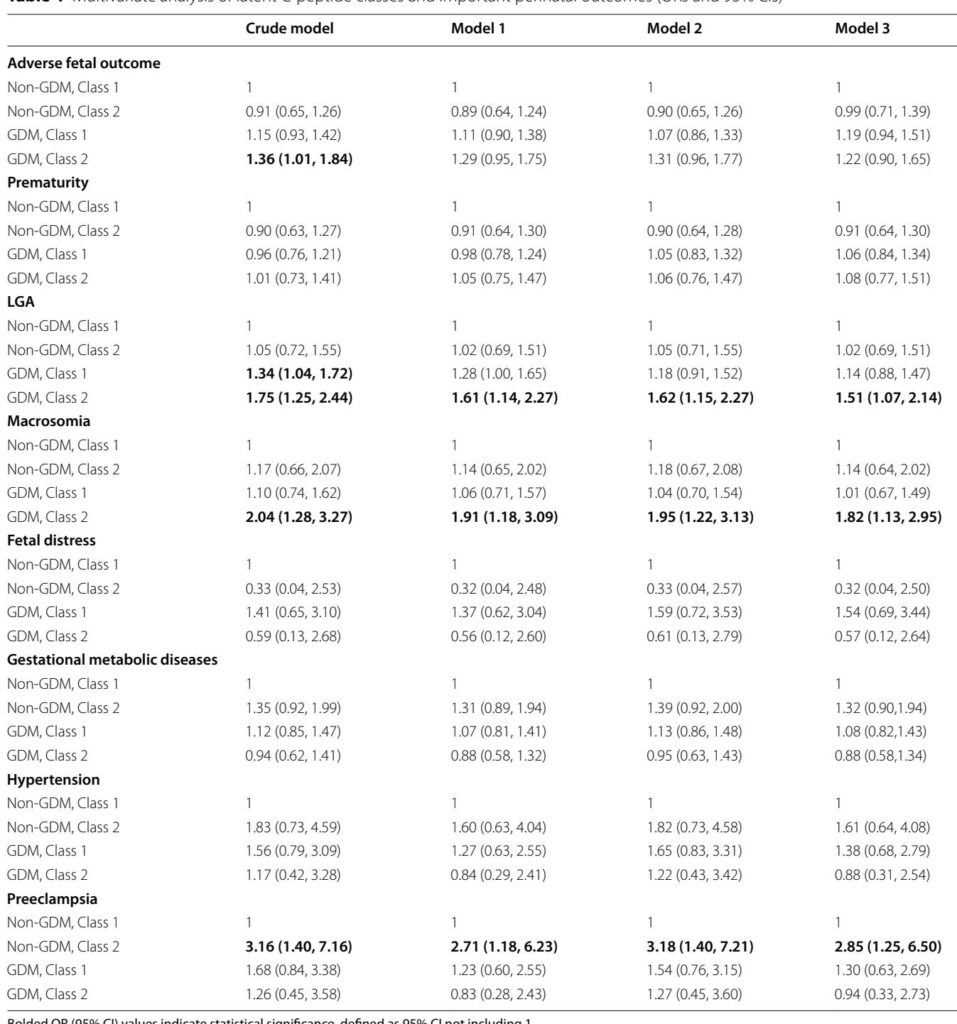
Two distinct C-peptide trajectory classes emerged within the GDM population despite similar glucose profiles. “GDM Class 1” (76.04% of GDM women) exhibited a delayed peak of C-peptide at 120 minutes with relatively lower insulin secretion indices, indicating beta-cell dysfunction. “GDM Class 2” (23.96%) showed a sharp C-peptide peak at 60 minutes followed by a decline, corresponding to marked insulin resistance demonstrated by elevated HOMA-IR and reduced insulin sensitivity indices.
Clinically, GDM Class 2 women were at significantly increased risk of delivering LGA neonates (adjusted odds ratio [aOR] 1.52; 95% CI 1.07–2.15) and macrosomia (aOR 1.83; 95% CI 1.13–2.97). Conversely, GDM Class 1 showed no significant independent associations after adjustment, suggesting a comparatively lower risk despite impaired secretion. Notably, 21.7% of normoglycemic women (non-GDM Class 2) exhibited a similar high C-peptide response with evidence of increased insulin resistance and were associated with elevated preeclampsia risk (aOR 2.91; 95% CI 1.25–6.74). These findings highlight subclinical metabolic dysregulation extending beyond current glucose-based diagnostic thresholds.
Demographic predictors of insulin-resistant classes included increased prepregnancy BMI, family history of diabetes, and prior cesarean delivery. The data underscore that incorporation of C-peptide trajectories can uncover latent metabolic subtypes in both GDM and normoglycemic pregnant populations, which informs risk of fetal overgrowth and hypertensive disorders respectively.
Expert Commentary
This study provides compelling pathophysiological insights, supporting the paradigm that GDM is a heterogeneous disorder comprising distinct metabolic phenotypes. The identification of a beta-cell deficient subgroup underscores the importance of recognizing impaired insulin secretion, while the insulin-resistant group represents a profile analogous to type 2 diabetes with hyperinsulinemia and risk of macrosomia. The observation of a high C-peptide insulin-resistant phenotype in normoglycemic women who develop preeclampsia challenges reliance solely on glucose thresholds, suggesting a spectrum of metabolic risk during pregnancy.
The application of LCTM to dynamic OGTT-derived C-peptide measurements is innovative, allowing for refined stratification without additional invasive testing beyond routine OGTT sampling. This stratification has potential to direct personalized therapeutic interventions—insulin sensitization and lifestyle modifications targeting Class 2, and beta-cell support and glycemic monitoring for Class 1. Further, identification of at-risk normoglycemic women could prompt early surveillance and preventive strategies for preeclampsia.
Limitations include a single-center, ethnically homogenous cohort and absence of long-term follow-up data to determine post-pregnancy metabolic trajectories. Validation in diverse populations and integration with genetic and mechanistic studies will augment clinical applicability.
Conclusions
Dynamic assessment of C-peptide during the OGTT elucidates the metabolic heterogeneity of GDM and identifies high-risk phenotypes for adverse pregnancy outcomes. The conventional glucose-only diagnostic approach obscures significant pathophysiological differences that have clinical relevance. Incorporation of C-peptide trajectories offers a pragmatic, noninvasive method to enhance risk stratification and individualize clinical management, potentially improving maternal and fetal outcomes in gestational diabetes and beyond.
References
1. Hartling L et al. Benefits and harms of treating gestational diabetes mellitus: a systematic review and meta-analysis. Ann Intern Med. 2013;159(2):123–9.
2. Wang H et al. IDF Diabetes Atlas: estimation of global and regional GDM prevalence. Diabetes Res Clin Pract. 2022;183:109050.
3. Saravanan P. Gestational diabetes: opportunities for improving maternal and child health. Lancet Diabetes Endocrinol. 2020;8(9):793–800.
4. Maddaloni E et al. C-peptide determination in the diagnosis of diabetes type and management: a clinical perspective. Diabetes Obes Metab. 2022;24(10):1912–26.
5. Gong Y et al. Heterogeneity of gestational diabetes and risk for adverse pregnancy outcome: a cohort study. J Clin Endocrinol Metab. 2025;110(7):e2264–72.
6. Matthews DR et al. Homeostasis model assessment: insulin resistance and beta-cell function. Diabetologia. 1985;28(7):412–9.
7. Matsuda M, DeFronzo RA. Insulin sensitivity indices from OGTT vs clamp. Diabetes Care. 1999;22(9):1462–70.