Highlights
The SHARP (Spincterotomy for Acute Recurrent Pancreatitis) trial represents the most rigorous evaluation to date of minor papillotomy for pancreas divisum. The study yielded several critical findings for the gastroenterology community:
- Minor papillotomy did not significantly reduce the risk of recurrent acute pancreatitis compared to a sham procedure (Adjusted Hazard Ratio 0.83; 95% CI, 0.49 to 1.41).
- No significant differences were observed in secondary outcomes, including the development of chronic calcific pancreatitis, diabetes, or exocrine pancreatic dysfunction.
- The frequency of acute pancreatitis episodes post-intervention remained comparable between the endoscopic treatment group and the sham control group.
- The procedure carried a notable risk, with post-ERCP pancreatitis occurring in 14.7% of the intervention group compared to 8.2% in the sham group.
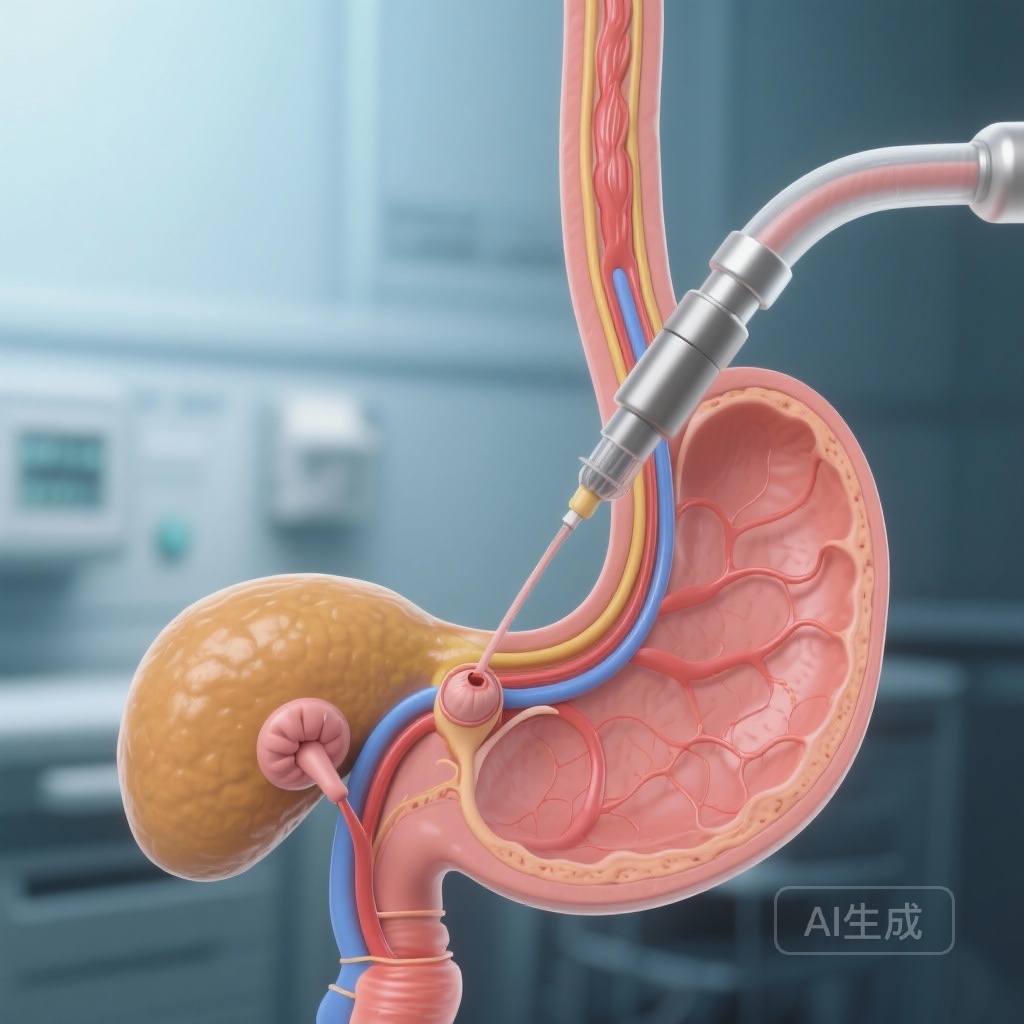
Introduction: The Pancreas Divisum Dilemma
Pancreas divisum is the most common congenital variant of pancreatic ductal anatomy, occurring in approximately 5% to 10% of the population. It results from the failure of the dorsal and ventral pancreatic buds to fuse during embryological development. Consequently, the majority of the pancreatic secretions must drain through the small minor papilla via the duct of Santorini, rather than through the larger major papilla. For decades, this anatomical configuration has been hypothesized to cause a relative outflow obstruction—often termed ‘dorsal duct hypertension’—which was believed to trigger idiopathic acute recurrent pancreatitis (ARP).
Based on this physiological premise, endoscopic retrograde cholangiopancreatography (ERCP) with minor papillotomy became a widely adopted intervention. By surgically or endoscopically enlarging the minor papilla, clinicians aimed to facilitate better drainage and prevent the inflammatory cascades leading to pancreatitis. While numerous observational studies and retrospective cohorts suggested a clinical benefit, these studies were frequently limited by selection bias, lack of control groups, and the inherent placebo effect associated with invasive procedures. The SHARP trial was designed to provide the definitive, high-quality evidence needed to support or refute this common practice.
Study Design and Methodology
The SHARP trial was a multicenter, sham-controlled, double-blind randomized clinical trial conducted across 21 referral centers in the United States and Canada. Between September 2018 and August 2024, the study enrolled 148 adults who met the following criteria: a history of two or more episodes of acute pancreatitis, documented pancreas divisum, and no other identifiable etiology for their pancreatitis (idiopathic ARP). Patients with established chronic calcific pancreatitis were excluded to ensure the study focused on the prevention of recurrent acute events rather than the management of end-stage disease.
Participants were randomized in a 1:1 ratio to either ERCP with minor papillotomy or a sham ERCP. To maintain blinding, the sham procedure involved sedation and the insertion of the duodenoscope to the level of the minor papilla, but without cannulation or sphincterotomy. Both the patients and the follow-up clinical teams remained unaware of the treatment assignment. The primary endpoint was the development of a subsequent episode of acute pancreatitis more than 30 days after randomization, analyzed as a time-to-event outcome. Secondary outcomes focused on long-term sequelae, including exocrine and endocrine insufficiency.
Key Findings: No Superiority Over Sham
The results of the SHARP trial, published in JAMA, provide a sobering look at the efficacy of minor papillotomy. Over a median follow-up period of 34 months, the study found that 34.7% (26 of 75) of participants in the minor papillotomy group experienced a recurrence of acute pancreatitis. In the sham group, 43.8% (32 of 73) experienced a recurrence. While the raw percentage was lower in the intervention group, the difference was not statistically significant, with an adjusted hazard ratio of 0.83 (95% CI, 0.49 to 1.41).
Furthermore, the frequency of episodes did not show a clinically meaningful divergence. The incidence rate ratio for episode frequency was 0.25 in the intervention group versus 0.30 in the sham group. When examining the progression to chronic disease, the data remained consistent: chronic calcific pancreatitis developed in 4.0% of the treatment group and 2.7% of the sham group. Similarly, rates of new-onset diabetes and exocrine pancreatic dysfunction showed no statistically significant differences between the two arms.
Safety and Procedural Risks
In addition to the lack of clear efficacy, the safety profile of the intervention warrants close attention. ERCP is inherently risky, and minor papilla cannulation is technically demanding. The trial reported that acute pancreatitis within 30 days of the procedure—largely considered post-ERCP pancreatitis (PEP)—occurred in 14.7% of those who underwent minor papillotomy. In contrast, the sham group, which did not undergo cannulation, had an 8.2% rate of pancreatitis within the same window. This suggests that the intervention not only fails to prevent future idiopathic episodes but also poses an immediate risk of triggering the very condition it aims to treat.
Expert Commentary: A Paradigm Shift in Gastroenterology
The SHARP trial follows in the footsteps of the EPISOD trial, which previously debunked the utility of biliary sphincterotomy for Type III Sphincter of Oddi Dysfunction. Both studies highlight a recurring theme in interventional medicine: procedures that seem physiologically sound and are supported by observational data often fail when subjected to the rigors of a sham-controlled, randomized trial.
Clinical experts suggest several reasons for these findings. First, the presence of pancreas divisum may be incidental in many patients with idiopathic ARP, rather than the primary driver. Genetic predispositions (such as mutations in CFTR, SPINK1, or PRSS1) may play a more significant role in these patients than anatomical variants. Second, the ‘obstruction’ theory may be overly simplistic; minor papillotomy might address the anatomy without resolving the underlying inflammatory or genetic susceptibility of the pancreatic tissue.
For clinicians, these results suggest that minor papillotomy should no longer be considered a first-line or routine treatment for patients with pancreas divisum and ARP. Instead, management should focus on aggressive lifestyle modifications (alcohol and smoking cessation), genetic counseling, and potentially medical therapies, while reserving endoscopic intervention for highly selected cases—perhaps those with objective evidence of dorsal duct dilation or failed medical management, although even then, the SHARP data suggests caution.
Conclusion
The SHARP trial provides high-level evidence that ERCP with minor papillotomy does not significantly reduce the risk of recurrent acute pancreatitis in patients with pancreas divisum. Given the lack of clear benefit and the documented risks of the procedure, these findings are likely to lead to a significant shift in clinical guidelines and a reduction in the use of minor papillotomy for this indication. The study underscores the vital importance of performing sham-controlled trials for endoscopic procedures to ensure that patients are not subjected to unnecessary risks for unproven benefits.
Funding and Clinical Trial Information
The SHARP trial was supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). The trial is registered at ClinicalTrials.gov with the identifier NCT03609944.
References
- Coté GA, Durkalski-Mauldin V, Fogel EL, et al. Minor Papillotomy for Treatment of Idiopathic Acute Pancreatitis With Pancreas Divisum: A Randomized Clinical Trial. JAMA. 2026;335(2):e2523988. doi:10.1001/jama.2025.23988.
- Cotton PB, Durkalski V, Paulus R, et al. Effect of endoscopic sphincterotomy for suspected sphincter of Oddi dysfunction on pain-related disability following cholecystectomy: the EPISOD randomized clinical trial. JAMA. 2014;311(20):2101-2109.
- Fogel EL, Toth TG, Lehman GA, et al. Does endoscopic minor papillotomy efficacious in patients with pancreas divisum and recurrent pancreatitis? Results of a 15-year retrospective study. Gastrointest Endosc. 2007;65(5):AB154.