Highlight
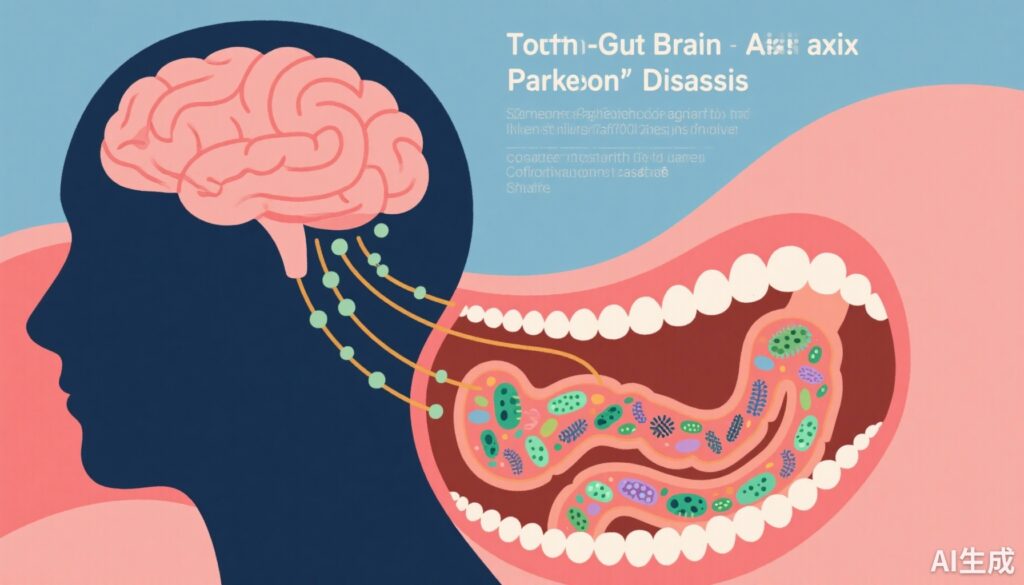
– Oral and gut microbiome virulence factors are linked to cognitive impairment progression in Parkinson’s disease (PD).
– Oral-gut translocation increases pathogenicity and disrupts host immunity and brain endothelial function.
– Integration of shotgun metagenomics and saliva metaproteomics reveals microbial functional dysregulation in PD mild cognitive impairment (PD-MCI) and dementia (PDD).
– Findings support the oral-gut-brain axis as a promising target for biomarkers and therapeutic intervention in neurodegeneration.
Study Background and Disease Burden
Parkinson’s disease (PD) affects over 10 million people worldwide, representing a major neurodegenerative disorder characterized predominantly by motor symptoms. However, non-motor symptoms, particularly cognitive impairment (CI), occur in the majority of PD patients throughout disease progression, ranging from mild cognitive impairment (PD-MCI) to dementia (PDD). The pathophysiological mechanisms underlying CI development in PD remain incompletely understood, creating significant challenges for early diagnosis and targeted interventions.
The human microbiome has emerged as a crucial factor influencing neurological health through the gut-brain axis. While the role of gut microbiota in neurodegeneration has received extensive attention, the oral microbiome and its interaction with the gut microbiome—termed oral-gut tropism—has been comparatively overlooked despite the oral cavity being a key reservoir of microbes capable of translocating to the gut. Dysbiosis involving pathobionts and virulence factors might trigger systemic inflammation, immune dysregulation, and blood-brain barrier (BBB) dysfunction, contributing to neurodegeneration and cognitive decline. Thus, exploring the oral-gut-brain axis offers a novel perspective on mechanisms driving CI in PD and identifies new opportunities for biomarker discovery and therapeutic targeting.
Study Design
This study by Clasen et al. employed a comprehensive multi-omics approach involving 228 shotgun metagenomics samples of the gut and oral microbiomes collected from PD patients stratified by cognitive status (PD-MCI and PDD) and a healthy control cohort. The oral and gut microbial communities were characterized in terms of taxonomic composition, functional gene pathways, and virulence signatures. Concurrently, saliva metaproteomics was integrated to assess microbial protein expression and their effects on host immunity and brain endothelial cell function.
The study design allowed comparison across cognitive impairment spectrum stages to identify microbial shifts correlating with disease progression. Main study endpoints included identification of microbial taxa, virulence factors, dysregulated metabolic pathways, and host-microbiome interaction features associated with PD-related cognitive decline.
Key Findings
Microbial Composition and Virulence Factors: Distinct differences in oral and gut microbiomes were observed between PD patients and controls, particularly in those with cognitive impairment. Pathobionts, microbial species with potential pathogenicity, were significantly enriched in PD-MCI and more so in PDD subjects. Notably, several oral-origin microbes were detected at increased abundance in the gut, demonstrating oral-gut translocation—a mechanism likely enhancing pathogenic potential.
Virulence factors, such as adhesion molecules, toxins, and immune evasion proteins, showed increased abundance correlating with cognitive decline severity. This enriches the concept that microbial virulence contributes actively to PD pathogenesis beyond simple dysbiosis.
Functional Dysregulation: Dysregulated metabolic pathways related to carbohydrate metabolism, amino acid metabolism, and neuroactive compound biosynthesis were identified in both gut and oral microbiomes, with exaggerated alterations in PDD. These pathways potentially influence neuroinflammatory and neurodegenerative processes.
Host-Microbiome Interactions: Integration with saliva metaproteomics revealed that elevated microbial virulence signatures coincided with host protein changes indicative of impaired immunity and blood-brain barrier endothelial dysfunction. This suggests that oral-gut microbes may exert direct effects on brain endothelial cells, promoting vascular permeability and facilitating neuroinflammatory cascades implicated in PD cognitive decline.
Additionally, the oral-gut-brain axis model proposed by the study underscores a dynamic interplay where oral pathobionts translocate to the gut, alter microbial ecology and virulence, and impact host systemic and neural environments.
Expert Commentary
This study represents a significant advance in understanding PD-associated cognitive decline by broadening the focus from the traditional gut-brain axis to include the oral microbiome and the oral-gut translocation pathway. The multi-omics integration strengthens the biological plausibility that microbial virulence factors contribute mechanistically to neurodegeneration and CI development through immune dysregulation and endothelial compromise. These insights challenge the notion that microbiome involvement is passive and suggest that targeted antimicrobial or microbiome modulation strategies could modify disease progression.
Limitations include the observational design that precludes causal inference and potential confounding by anti-PD medications, diet, and oral hygiene. Future longitudinal cohorts and mechanistic experimental models are needed to validate these findings, dissect causal pathways, and translate biomarker candidates into clinical practice.
Conclusion
In summary, the oral and gut microbiomes are integral components of the neurodegenerative process in PD, particularly influencing cognitive decline through increased virulence factor expression and oral-gut microbial translocation. This study highlights the oral-gut-brain axis as a promising frontier for elucidating PD pathophysiology, developing early biomarkers for cognitive impairment, and designing microbiome-targeted therapeutics. Clinicians should be aware of the multidimensional microbiome contributions to PD non-motor symptoms, and ongoing research should integrate oral health and microbiome assessments in PD management.
References
- Clasen F, Yildirim S, Arıkan M, et al. Microbiome signatures of virulence in the oral-gut-brain axis influence Parkinson’s disease and cognitive decline pathophysiology. Gut Microbes. 2025 Dec;17(1):2506843. doi: 10.1080/19490976.2025.2506843.
- Braak H, Del Tredici K. Neuroanatomy and pathology of sporadic Parkinson’s disease. Adv Anat Embryol Cell Biol. 2009;201:1-119.
- Mulak A, Bonaz B. Brain-gut-microbiota axis in Parkinson’s disease. World J Gastroenterol. 2015 Aug 14;21(37):10609-20.
- Heintz-Buschart A, Wilmes P. Human gut microbiome: function matters. Trends Microbiol. 2018 Jul;26(7):563-574.
- Needham BD, Trent MS. Fortifying the barrier: the impact of lipopolysaccharide modification on bacterial pathogenesis. Nat Rev Microbiol. 2013 Jul;11(7):467-81.