Highlights
- The C-GUARDIANS trial demonstrates a primary endpoint (30-day DSMI and 1-year ipsilateral stroke) incidence of only 1.93%.
- The 30-day rate of death, stroke, or myocardial infarction (DSMI) was 0.95%, representing a significant safety profile in high-risk patients.
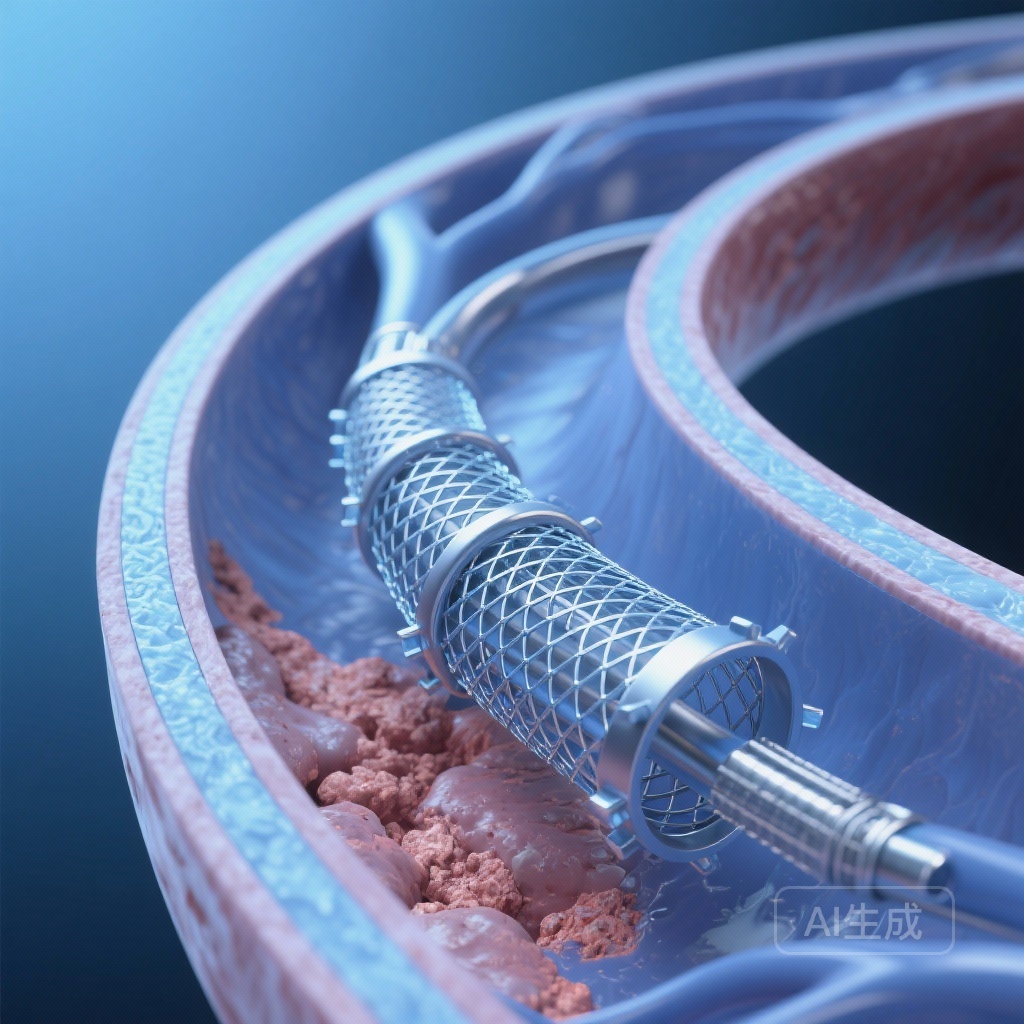
- The MicroNet-covered stent technology effectively addresses the embolic window by preventing plaque protrusion through the stent struts.
- Target lesion revascularization (TLR) at one year remained exceptionally low at 1.0%.
The Evolution of Carotid Artery Stenting and the Persistent Embolic Challenge
For decades, carotid endarterectomy (CEA) has been the gold standard for the treatment of significant carotid artery stenosis. While carotid artery stenting (CAS) emerged as a less invasive alternative, its adoption was historically tempered by concerns regarding peri-procedural embolic events. Traditional open-cell and closed-cell stents, while effective at maintaining vessel patency, often fail to contain friable atherosclerotic debris. This debris can protrude through the stent struts—a phenomenon known as plaque prolapse—leading to distal embolization and subsequent neurological events both during and after the procedure.
The ’embolic window’—the period following stent deployment but preceding full endothelialization—remains a critical vulnerability in CAS. To address this, the medical community has sought innovations that provide continuous embolic protection. The C-GUARDIANS trial investigates the CGuard MicroNet-covered stent, a novel system designed to bridge this gap by utilizing a polyethylene terephthalate (PET) mesh to provide a permanent barrier against plaque protrusion.
Study Design and Patient Population
The C-GUARDIANS (Safety and Efficacy of the CGuard Carotid Stent System in Carotid Artery Stenting) trial was a prospective, multicenter, single-arm, pivotal trial (NCT04900844). Conducted across 24 sites in the United States and the European Union, the study enrolled 316 patients between July 2021 and June 2023. The cohort consisted of patients with significant carotid stenosis (asymptomatic ≥80% or symptomatic ≥50%) who were considered at high risk for complications from traditional carotid endarterectomy.
The primary endpoint was a composite of the incidence of death, all stroke, and myocardial infarction (DSMI) through 30 days post-procedure, combined with the incidence of ipsilateral stroke from day 31 to day 365. Secondary endpoints focused on individual components of the composite endpoint at 30 days and one year, as well as the technical success of the procedure and the rate of target lesion revascularization (TLR). All clinical events were adjudicated by an independent clinical events committee to ensure rigorous data integrity.
The Technology: How MicroNet Changes the Paradigm
The CGuard system differs from conventional stents through its dual-layer design. It features a nitinol frame for structural support, covered by a MicroNet—a PET mesh with a pore size of approximately 150-180 μm. This ultra-fine mesh is designed to trap potential emboli against the vessel wall, preventing them from entering the bloodstream. Unlike traditional stents where the ‘free cell area’ can be as large as 10 mm², the MicroNet reduces this area significantly, theoretically providing superior neuroprotection without compromising the flexibility or radial strength required for carotid interventions.
Key Findings: Safety and Efficacy Results
The results of the C-GUARDIANS trial provide compelling evidence for the safety and durability of the MicroNet system. Of the 316 patients in the intention-to-treat population, the 30-day DSMI rate was a remarkably low 0.95% (3/316). This is particularly noteworthy given the high-risk nature of the study population.
At the one-year follow-up mark, the composite primary endpoint (30-day DSMI plus 1-year ipsilateral stroke) was observed in only 1.93% of patients (6/296). Furthermore, the incidence of target lesion revascularization was only 1.0% (3/299), suggesting that the presence of the MicroNet does not increase the risk of in-stent restenosis or require frequent secondary interventions. The trial reported no unexpected adverse device effects or serious adverse device effects, confirming the biocompatibility and mechanical stability of the PET mesh over the long term.
Statistical Significance and Clinical Context
When compared to historical data from pivotal CAS trials such as CREST or ACT I, the 0.95% 30-day DSMI rate in C-GUARDIANS stands out as exceptionally low. While cross-trial comparisons must be interpreted with caution due to differences in patient selection and embolic protection device (EPD) usage, the C-GUARDIANS data suggests that mesh-covered stents may significantly narrow the safety gap between CAS and CEA, even in high-risk surgical candidates.
Expert Commentary: A Shift Toward ‘Bio-Inductive’ Neuroprotection
Clinical experts have noted that the MicroNet technology represents a shift from simple mechanical scaffolding to a more sophisticated form of neuroprotection. By providing a ‘shield’ against the embolic material, the stent addresses the limitation of distal embolic protection filters, which are only present during the procedure itself. The CGuard system provides protection during the high-risk post-procedural period when the plaque is most unstable.
However, some clinicians emphasize that while the results are excellent, the single-arm nature of the trial is a limitation. Future randomized controlled trials comparing mesh-covered stents directly against traditional stents or transcarotid artery revascularization (TCAR) would provide more definitive evidence regarding the superiority of this specific mesh design. Additionally, the long-term behavior of the PET mesh beyond one year remains an area of ongoing observation, though the current TLR rates are highly encouraging.
Conclusions and Future Directions
The C-GUARDIANS trial successfully demonstrated that the CGuard MicroNet-covered stent is safe, effective, and durable for the treatment of carotid artery stenosis in patients at high risk for CEA. The exceptionally low rates of peri-procedural events and the sustained protection against ipsilateral stroke through one year support the hypothesis that mesh-covered stents can provide superior neuroprotection by preventing plaque protrusion.
As the field of vascular intervention continues to move toward minimally invasive solutions, technologies like MicroNet are likely to play a central role in expanding the reach of CAS to a broader patient population, potentially including those who were previously deferred due to high embolic risk. The C-GUARDIANS results provide a robust foundation for the clinical adoption of this novel stent system in both the United States and Europe.
Funding and Clinical Trial Information
The C-GUARDIANS trial was sponsored by InspireMD. ClinicalTrials.gov Identifier: NCT04900844.
References
Metzger DC, Soukas P, Siddiqui A, et al. Safety and Efficacy of a Novel Micro Net Carotid Stent System: Results of the C-GUARDIANS Trial. JACC Cardiovasc Interv. 2025;18(24):3087-3097. doi:10.1016/j.jcin.2025.09.027.