Highlights
Patients who achieved guideline-recommended low-density lipoprotein cholesterol (LDL-C) levels following ST-segment elevation myocardial infarction (STEMI) demonstrated a significantly lower prevalence of neoatherosclerosis (7%) compared to those who did not reach these targets (19%).
Multivariable analysis revealed that for every 25-mg/dL increase in on-treatment LDL-C levels, the risk of developing neoatherosclerosis within three years of drug-eluting stent (DES) implantation increased by 46%.
The use of optical coherence tomography (OCT) provided high-resolution evidence that systemic lipid control directly influences the microscopic environment of the stented coronary segment.
These findings reinforce the ‘lower is better’ hypothesis for LDL-C, extending its benefits from native vessel plaque stabilization to the prevention of late stent failure mechanisms.
Background: The Challenge of Late Stent Failure
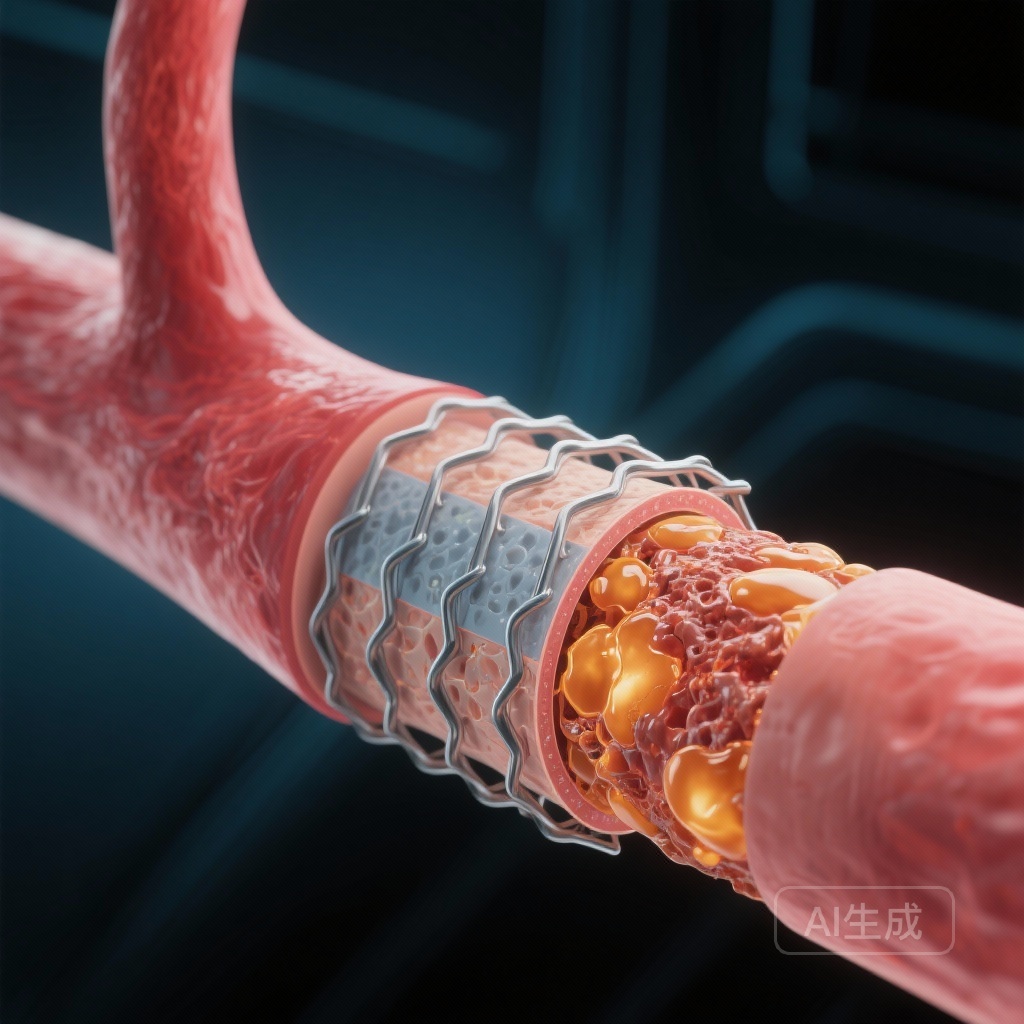
Despite the revolutionary impact of drug-eluting stents (DES) on reducing early restenosis and target lesion revascularization, the long-term management of stented segments remains a significant clinical hurdle. Neoatherosclerosis—the development of a new atherosclerotic plaque within the neointimal layer of a stented segment—has emerged as a primary driver of late and very late stent failure. Unlike traditional restenosis, which is characterized by smooth muscle cell proliferation, neoatherosclerosis involves lipid-laden macrophage infiltration, fibroatheroma formation, and potentially, plaque rupture and intra-stent thrombosis.
In the context of ST-segment elevation myocardial infarction (STEMI), where the inflammatory milieu is heightened and the risk of future cardiovascular events is elevated, secondary prevention is paramount. While the association between low-density lipoprotein cholesterol (LDL-C) and the progression of native coronary artery disease is well-established, its specific role in the formation of neoatherosclerosis within modern DES platforms has remained less clear. Understanding this relationship is critical for optimizing post-PCI pharmacological therapy and improving long-term patient outcomes.
Study Design and Methodology
The present study is a post hoc analysis of the CONNECT (Comparison of Neoatherosclerosis Between Biolimus-Eluting and Everolimus-Eluting Stents) randomized clinical trial. Conducted across seven high-volume cardiac centers in Switzerland and Japan, the trial originally randomized 239 patients with STEMI to undergo primary percutaneous coronary intervention (PCI) with either biodegradable-polymer or durable-polymer everolimus-eluting stents between 2017 and 2020.
For this secondary analysis, researchers focused on 178 patients who survived to three years and underwent follow-up optical coherence tomography (OCT). OCT is considered the gold standard for intravascular imaging due to its near-histological resolution (10–20 μm), allowing for the precise identification of neoatherosclerotic features such as lipid-rich neointima, macrophage accumulation, and calcification.
Patients were categorized into two groups based on whether they achieved guideline-endorsed target LDL-C levels (typically defined as <55 mg/dL or a ≥50% reduction from baseline, depending on specific regional guidelines at the time of the study). Statin therapy and other lipid-lowering agents were prescribed according to the standard of care. The primary endpoint was the prevalence of neoatherosclerosis at the 3-year mark, as assessed by independent core laboratory imaging analysis.
Key Findings: The Protective Effect of Low LDL-C
Among the 178 patients evaluated at three years, the mean age was 63.4 years, and 15% were female. The cohort was split into those who achieved the target LDL-C level (n = 98, 55%) and those who did not (n = 80, 45%). The disparity in lipid control was evident: the mean on-treatment LDL-C level in the target group was 48 mg/dL, while the non-target group averaged 87 mg/dL.
Prevalence of Neoatherosclerosis
The results were striking. Neoatherosclerosis was detected in only 7% of patients who achieved their target LDL-C levels, compared to 19% of those who remained above the target. This translated to a three-fold increase in the risk of neoatherosclerosis for patients with suboptimal lipid control (Odds Ratio [OR], 3.00; 95% CI, 1.19-8.24; P = .02).
Multivariable Predictor Analysis
To account for potential confounders, researchers performed a multivariable logistic regression analysis. The on-treatment LDL-C level emerged as a powerful and independent determinant of neoatherosclerosis formation. Specifically, for every 25-mg/dL increase in LDL-C, the risk of neoatherosclerosis rose by 46% (OR, 1.46; 95% CI, 1.09-2.01; P = .01). Other factors, such as stent type (biodegradable vs. durable polymer) or baseline patient characteristics, did not show the same level of independent predictive power for this specific outcome at the three-year mark.
Morphological Characteristics
OCT imaging revealed that the neoatherosclerotic lesions in the high-LDL group were more likely to exhibit characteristics associated with plaque instability. While the study was not powered to detect differences in rare clinical events like late stent thrombosis, the higher prevalence of lipid-rich neointima in the non-target group suggests a higher substrate for future acute coronary syndromes.
Expert Commentary: Mechanistic Insights and Clinical Implications
The findings from the CONNECT trial analysis provide a crucial link between systemic metabolic health and local vascular healing. The development of neoatherosclerosis is thought to be accelerated in DES compared to bare-metal stents because the drug-polymer coating can delay functional endothelialization. This ‘leaky’ or dysfunctional endothelium allows for the easier transmigration of circulating LDL-C into the sub-endothelial space of the neointima.
Once inside the neointima, LDL-C undergoes oxidation and is taken up by macrophages, leading to foam cell formation. Because the neointima in a stent is a relatively sequestered environment with altered shear stress, this process can occur much more rapidly than native atherosclerosis—sometimes within months to a few years rather than decades. By maintaining extremely low levels of circulating LDL-C (approaching 40-50 mg/dL), clinicians are essentially reducing the ‘pressure’ or gradient that drives lipid infiltration into the vessel wall.
From a clinical perspective, this study reinforces the necessity of the ‘statin-plus’ approach. Given that 45% of patients in this trial failed to meet target levels despite being in a controlled trial environment, there is a clear need for earlier and more aggressive use of non-statin therapies, such as ezetimibe and PCSK9 inhibitors, particularly in the high-risk STEMI population. We can no longer view stent implantation as a ‘fix’ for a localized problem; rather, it is a catalyst that requires even more stringent systemic risk factor modification.
Limitations and Future Directions
While the data are compelling, several limitations must be noted. As a post hoc analysis, the study was not originally powered specifically for the neoatherosclerosis endpoint. Furthermore, the 3-year follow-up, while significant, may not capture the full trajectory of neoatherosclerosis, which continues to increase in prevalence over 5 to 10 years. Additionally, the cohort was primarily Japanese and Swiss, which may limit the generalizability to other ethnic groups with different baseline metabolic profiles.
Future research should focus on whether ultra-low LDL-C levels (e.g., <30 mg/dL) can not only prevent but potentially regress early neoatherosclerotic changes. Moreover, the integration of artificial intelligence in OCT analysis could provide more automated and standardized assessments of neoatherosclerosis in larger clinical registries.
Conclusion
The secondary analysis of the CONNECT randomized clinical trial provides robust evidence that on-treatment LDL-C levels are a primary driver of neoatherosclerosis after DES implantation for STEMI. Achieving guideline-recommended lipid targets is associated with a dramatic reduction in the formation of new, high-risk plaques within the stent. These results underscore that aggressive lipid-lowering therapy is not just a tool for preventing native disease progression, but a fundamental requirement for ensuring the long-term structural and functional integrity of coronary stents.
Funding and Trial Information
The CONNECT trial was supported by various academic and clinical grants. ClinicalTrials.gov Identifier: NCT03440801. Data analysis was conducted between September 2024 and October 2025.
References
1. Häner JD, Kakizaki R, Taniwaki M, et al. Low-Density Lipoprotein Cholesterol Levels and Neoatherosclerosis After STEMI: A Secondary Analysis of the CONNECT Randomized Clinical Trial. JAMA Cardiol. 2025; Published online December 17, 2025. doi:10.1001/jamacardio.2025.4723.
2. Otsuka F, et al. Neoatherosclerosis: an overview of the histopathologic findings and implications for stent failure. Eur Heart J. 2015;36(32):2147-2159.
3. Mach F, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41(1):111-188.
4. Nakazawa G, et al. The pathology of neoatherosclerosis in human coronary implants bare-metal and drug-eluting stents. J Am Coll Cardiol. 2011;57(11):1314-1322.