Highlights
– Multiple experimental models and human aortic samples show up-regulation of the hexosamine biosynthetic pathway (HBP) and increased glycosylation in thoracic aortic aneurysm and dissection (TAAD).
– HBP activation promotes vascular smooth muscle cell (VSMC) dysfunction and engages the integrated stress response (ISR), driving medial degeneration and aortic dilatation.
– Pharmacologic inhibition of HBP or ISR reverses molecular, cellular, and imaging features of disease in a Marfan syndrome mouse model, identifying a potential therapeutic axis for genetic and sporadic TAAD.
Background and disease burden
Thoracic aortic aneurysms and dissections (TAADs) are life‑threatening structural diseases of the aortic wall characterized by progressive medial degeneration, extracellular matrix remodeling, and loss of functional vascular smooth muscle cells. TAADs arise in both inherited connective tissue disorders, most notably Marfan syndrome from FBN1 mutations, and in sporadic disease associated with ageing, hypertension, and unidentified multifactorial contributors. Management remains largely surgical for imminent or established aneurysm because no medical therapy reliably halts progression in most patients. Identifying modifiable molecular drivers that act across genetic and non‑genetic forms of TAAD is therefore an urgent unmet need.
Study design and methods
The work by Rochano‑Ortiz and colleagues used an integrative, translational approach combining preclinical models and human tissue analysis to test whether metabolic changes in glycan synthesis promote TAAD. The authors examined HBP activation using transcriptomics and metabolomics in three contexts: an established Marfan syndrome (MFS) mouse model, a non‑genetic TAAD model induced by β‑aminopropionitrile (BAPN; a lysyl oxidase inhibitor that induces medial degeneration), and aortic tissue from patients with sporadic TAAD and from those with MFS.
Key experimental readouts included aortic ultrasound imaging to assess dilatation, histopathology to quantify medial degeneration and glycan accumulation, VSMC functional assays, and molecular profiling to detect HBP metabolites and ISR activation. To assess causality and therapeutic potential the authors applied pharmacologic inhibitors of HBP activity and of the ISR in the MFS mouse model and evaluated structural and molecular endpoints.
Key findings
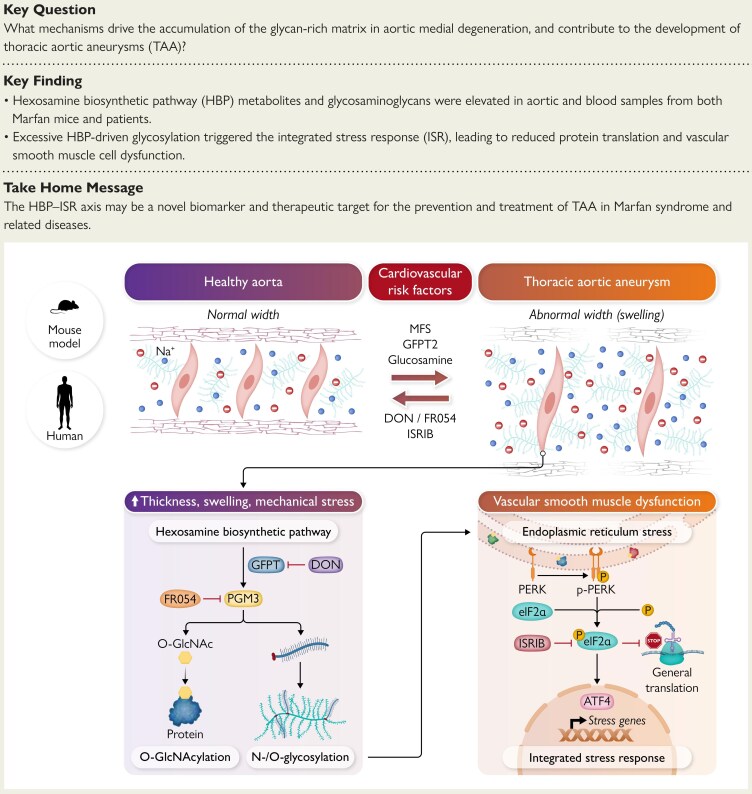
The central observation is a consistent up‑regulation of the hexosamine biosynthetic pathway in diseased aorta across models and human patients. Specific findings include:
- Transcriptomic signatures indicative of increased HBP enzyme expression in MFS and BAPN models and in human TAAD samples.
- Metabolomic evidence of elevated HBP flux, reflected by increased levels of pathway metabolites such as UDP‑GlcNAc, consistent with enhanced cellular glycosylation potential.
- Histologic accumulation of glycan‑rich extracellular matrix components within the medial layer, coinciding with markers of VSMC phenotypic change and loss.
- Activation of the integrated stress response, demonstrated by up‑regulation of canonical ISR effectors. The ISR is a conserved cellular program responding to proteostatic and metabolic stress that can shift gene expression and protein synthesis to manage damage but may promote maladaptive remodeling in chronic activation.
- In vitro and ex vivo analyses indicating that HBP hyperactivity induced maladaptive VSMC behavior, including altered contractile marker expression, increased synthetic phenotype features, and decreased resilience to stressors.
- Most importantly, targeted pharmacologic inhibition of HBP or pharmacologic attenuation of the ISR in the MFS mouse model led to measurable benefits: reduced aortic dilatation on ultrasound, improved medial architecture on histology, normalization of VSMC markers, and reduced ISR signaling.
Together these results support a mechanistic sequence in which excess HBP flux increases protein glycosylation and extracellular glycan deposition, triggering cellular stress pathways including the ISR, which then drives VSMC dysfunction and medial degeneration culminating in aneurysm formation.
Clinical and translational implications
These data shift attention toward metabolic and post‑translational modification pathways as drivers of aortic disease. Several implications follow:
- HBP and ISR represent convergent nodes through which diverse upstream insults might cause medial degeneration. That the HBP–ISR axis is active in both genetic MFS and sporadic human TAAD samples raises the possibility of a shared, targetable disease mechanism.
- Pharmacologic targeting of metabolism or stress responses could complement current risk factor management and potentially slow progression in patients for whom surgery is not yet indicated. Candidate agents used in preclinical work include small‑molecule inhibitors of HBP flux and ISR modulators. However, these agents are largely experimental and have not been proven safe or effective in TAAD patients.
- Future therapies may require tissue targeting to avoid systemic toxicity because HBP and ISR are fundamental in multiple organs and physiologic responses. Biomarkers of HBP activity, such as tissue or circulating UDP‑GlcNAc levels or measures of protein O‑GlcNAcylation, could facilitate patient selection and pharmacodynamic monitoring.
Expert commentary and mechanistic considerations
Strengths of the study include the use of complementary disease models, integration of human tissue data, and a combination of transcriptomic and metabolomic methods that provide convergent evidence for HBP activation. Demonstrating that pharmacologic blockade reverses disease features in a genetic model strengthens the argument for causality and therapeutic potential.
Important mechanistic points and limitations to consider:
- Biology of glycosylation. The HBP produces UDP‑GlcNAc, the donor for multiple glycosylation reactions including O‑GlcNAcylation, N‑glycosylation of secreted proteins, and glycosaminoglycan synthesis. These modifications affect intracellular signaling, extracellular matrix composition, and protein folding in the endoplasmic reticulum, any of which could plausibly alter VSMC function and matrix integrity.
- ISR activation may be downstream of ER stress provoked by altered glycosylation or by accumulation of misfolded proteins. The ISR has adaptive roles in acute stress but can become maladaptive when chronically engaged. Targeting the ISR therefore needs caution because of potential interference with protective stress responses.
- Pharmacology and off‑target effects. Small molecules that inhibit HBP enzymes or modulate ISR signaling are not yet licensed for chronic cardiovascular use. Many HBP inhibitors affect amino acid metabolism broadly, and ISR modulators can alter global protein synthesis. Long‑term safety, dose selection, and delivery to the aortic wall remain unanswered.
- Translation from models to humans. Mouse models, including Fbn1 mutant mice recapitulating Marfan features and BAPN‑induced aneurysm, are valuable but imperfect. Human TAAD is heterogeneous. The presence of HBP activation in patient samples is encouraging, but prospective clinical data linking activity to clinical progression or response to intervention are required.
Path forward: research and clinical development priorities
To translate these findings into clinical impact, several steps are needed:
- Replication and extension in additional human cohorts with longitudinal sampling to test whether HBP/ISR markers predict aneurysm growth or dissection risk.
- Refinement of pharmacologic strategies, including identification or development of agents with favorable specificity and safety profiles, and strategies for local delivery to minimize systemic exposure.
- Preclinical chronic dosing studies evaluating safety, vascular selectivity, and effects on blood pressure, wound healing, infection risk, and other ISR‑dependent processes.
- Early‑phase clinical trials in well‑phenotyped patient groups, such as patients with genetically confirmed Marfan syndrome and measurable progressive thoracic aortic dilatation, using imaging and biomarker endpoints to assess target engagement and biological activity before moving to large outcome trials.
- Investigation of potential interactions with established medical therapies for aortic disease, including β‑blockers, angiotensin receptor blockers, and blood pressure control strategies.
Conclusion
The study by Rochano‑Ortiz et al. identifies the hexosamine biosynthetic pathway and integrated stress response as a mechanistic axis driving medial degeneration in thoracic aortic aneurysm across genetic and non‑genetic contexts. The work advances a provocative model in which excessive glycosylation perturbs VSMC homeostasis and extracellular matrix composition, engaging conserved stress pathways that culminate in aneurysm formation. Preclinical reversal of disease features by inhibiting HBP or ISR supports the concept that metabolism and proteostatic signaling are actionable disease nodes. Translation to clinical practice will require careful pharmacologic development, biomarker‑guided clinical studies, and attention to safety given the central roles these pathways play in normal physiology.
Funding and clinicaltrials.gov
Clinical trials targeting HBP or ISR in TAAD have not yet been registered at clinicaltrials.gov at the time of publication of the cited work. The cited study reports preclinical and human tissue data supporting future trial development. Any clinical development program must include robust safety evaluation given systemic roles of glycosylation and stress signaling.
References
1. Rochano‑Ortiz A, San Sebastián‑Jaraba I, Zamora C, Simó C, García‑Cañas V, Martínez‑Albaladejo S, Fernández‑Gómez MJ, et al. Excessive glycosylation drives thoracic aortic aneurysm formation through integrated stress response. Eur Heart J. 2025 Dec 1;46(45):4988‑5005. doi: 10.1093/eurheartj/ehaf556 IF: 35.6 Q1 . PMID: 40720766 IF: 35.6 Q1 ; PMCID: PMC12665370 IF: 35.6 Q1 .
2. Loeys BL, Dietz HC, Braverman AC, Callewaert BL, De Backer J, Devereux RB, et al. The revised Ghent nosology for the Marfan syndrome. J Med Genet. 2010 Jul;47(7):476‑85.
Note: Additional foundational literature on HBP, protein O‑GlcNAcylation, and ISR biology is extensive and should be consulted when designing translational studies. The references listed are focused on the primary translational report and clinical context for Marfan syndrome.