Highlight
– Early TAVR intervention outperforms surveillance even in asymptomatic severe aortic stenosis patients.
– Elevated NT-proBNP and hs-cTnT levels correlate with higher adverse event rates.
– Contrary to expectations, patients with lower baseline hs-cTnT benefit more from early TAVR.
– Single biomarker measurements have limited utility in timing TAVR for asymptomatic patients.
Study Background and Disease Burden
Aortic stenosis (AS) is a progressive valvular heart disease characterized by narrowing of the aortic valve, leading to increased left ventricular afterload and maladaptive cardiac remodeling. Severe AS without symptoms poses clinical management challenges, as the optimal timing of intervention remains debated. The EARLY TAVR trial addresses this gap by assessing whether early transcatheter aortic valve replacement (TAVR) offers improved clinical outcomes compared to watchful waiting and delayed intervention in asymptomatic severe AS patients. Cardiac biomarkers such as NT-proBNP and high-sensitivity cardiac troponin T (hs-cTnT) reflect myocardial stress and injury, respectively, and have established prognostic value in symptomatic AS. However, their role in guiding management for asymptomatic severe AS is uncertain.
Study Design
The EARLY TAVR trial was a randomized controlled study enrolling 901 patients with asymptomatic severe high-gradient aortic stenosis. Participants were randomized to receive either early TAVR or clinical surveillance with TAVR postponed until symptom onset or guideline-based indication. Biospecimens for measuring NT-proBNP and hs-cTnT were obtained from 798 patients (89% of the cohort) and analyzed in a core laboratory. The study’s primary endpoint was a composite of death, stroke, or unplanned cardiovascular hospitalization. Secondary endpoints included individual components of the composite and heart failure hospitalizations. Kaplan-Meier survival analyses and Cox proportional hazards models evaluated associations between biomarker levels and clinical outcomes. Interaction analyses tested whether baseline biomarker concentrations modified the efficacy of early TAVR.
Key Findings
Median biomarker levels were 287 pg/mL for NT-proBNP and 14.6 ng/L for hs-cTnT. Higher levels of both biomarkers were associated with increased risks for multiple adverse outcomes, consistent with their pathophysiological roles in myocardial stress and damage.
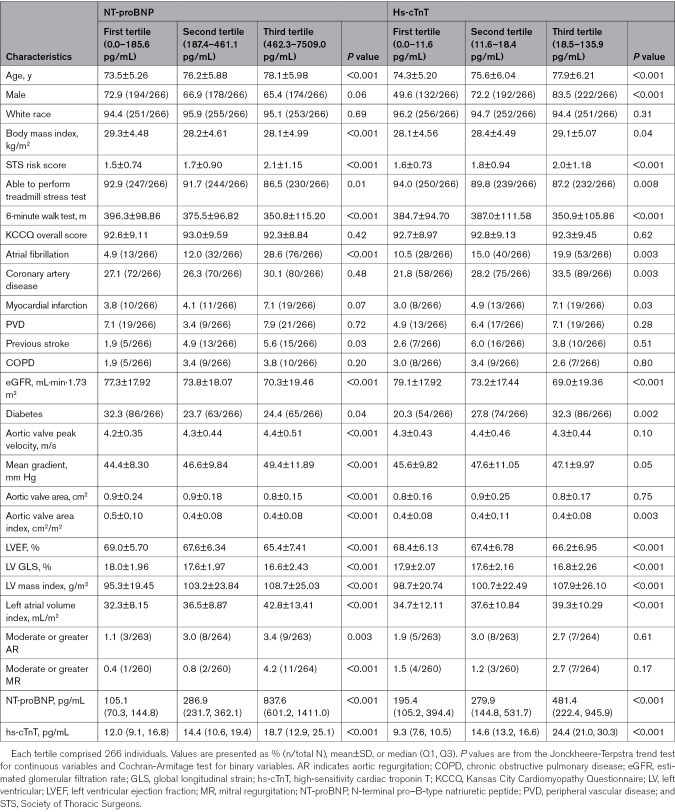
Table 1. Baseline Characteristics by Screening NT-proBNP and hs-cTnT Level
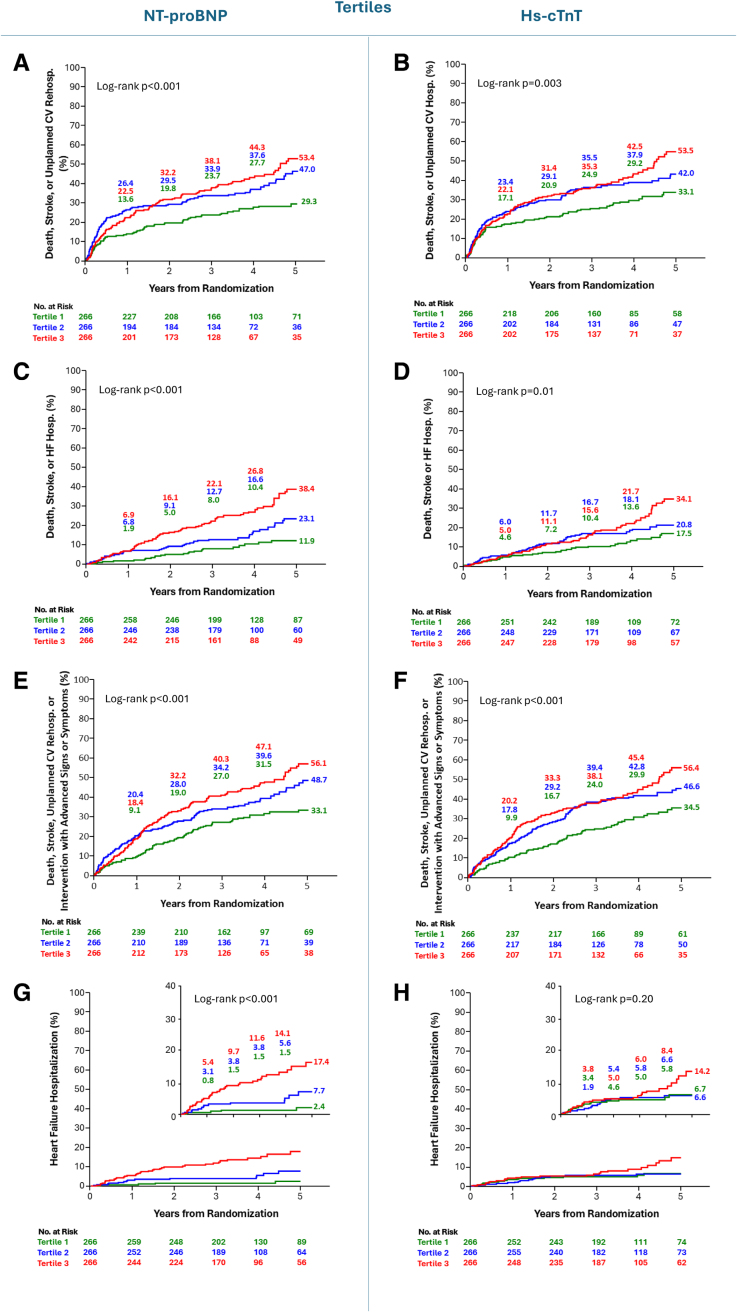
Notably, interaction testing revealed no significant modification of early TAVR treatment effect by baseline NT-proBNP or hs-cTnT levels for most endpoints. However, for the composite of death or heart failure hospitalization and heart failure hospitalization alone, a statistically significant interaction emerged with hs-cTnT levels (P=0.04 and P=0.03, respectively). The relative benefit of early TAVR was greater in patients with normal hs-cTnT compared to those with elevated levels at baseline.
Fig. Kaplan-Meier curves for the key clinical outcomes according to NT-proBNP and hs-cTnT tertiles.
Furthermore, while patients with higher baseline NT-proBNP exhibited a numerically larger absolute risk reduction from early TAVR, this did not translate into a stronger relative benefit. Trends suggested early TAVR’s advantage was broadly consistent regardless of biomarker levels.
These findings challenge the hypothesis that elevated cardiac biomarkers in asymptomatic severe AS identify patients who derive the greatest relative benefit from early intervention. Instead, those with lower biomarker levels appeared to experience more pronounced relative improvements.
Expert Commentary
This analysis provides valuable evidence on the prognostic and predictive roles of NT-proBNP and hs-cTnT in asymptomatic severe AS management. While these biomarkers robustly associate with adverse events, their utility as standalone tools to optimize TAVR timing is limited. The apparent paradox—greater relative benefit in patients with lower baseline cardiac injury markers—may reflect earlier myocardial reserve and less irreversible damage, underscoring the complexity of AS pathophysiology.
Limitations include reliance on a single baseline biomarker measurement without serial assessment and exclusion of other emerging biomarkers or imaging modalities that could augment risk stratification. The study’s strength lies in its rigorous design, large sample, and comprehensive biomarker evaluation within a randomized trial framework, enhancing the validity and clinical applicability of results.
Current guidelines recommend symptom-driven intervention for severe AS, with emerging data supporting earlier TAVR. This study advances understanding but signals caution in overrelying on isolated biomarker values for procedural timing.
Conclusion
In asymptomatic patients with severe high-gradient aortic stenosis, elevated NT-proBNP and hs-cTnT levels predict higher cardiovascular risk but do not identify those who benefit more from early TAVR. The relative advantage of early intervention is consistent across biomarker strata, with a surprising trend favoring those with lower hs-cTnT levels. These findings suggest that decisions on timing of TAVR should not be based solely on single biomarker measurements. Comprehensive clinical evaluation remains essential to optimize patient outcomes.
References
Lindman BR, Pibarot P, Schwartz A, Oldemeyer JB, Su YR, Goel K, Cohen DJ, Fearon WF, Babaliaros V, Daniels D, Chhatriwalla A, Suradi HS, Shah P, Szerlip M, Mack MJ, Dahle T, O’Neill WW, Davidson CJ, Makkar R, Sheth T, Depta J, DeVries JT, Southard J, Pop A, Sorajja P, Hahn RT, Zhao Y, Leon MB, Généreux P; EARLY TAVR Trial Executive Committee and Study Investigators. Cardiac Biomarkers in Patients With Asymptomatic Severe Aortic Stenosis: Analysis From the EARLY TAVR Trial. Circulation. 2025 Jun 3;151(22):1550-1564. doi: 10.1161/CIRCULATIONAHA.125.074425 IF: 38.6 Q1 . Epub 2025 Mar 31. PMID: 40163596 IF: 38.6 Q1 ; PMCID: PMC12124205 IF: 38.6 Q1 .
ClinicalTrials.gov. Evaluation of TAVR Compared to Surveillance for Patients With Asymptomatic Severe Aortic Stenosis. NCT03042104. https://www.clinicaltrials.gov/ct2/show/NCT03042104