Highlights
- The P/G@IYCe dressing achieved a 73.3% wound closure rate by day 14 in an S. aureus-infected model, significantly outperforming control groups.
- Dual delivery of IgY and CeO2 nanoparticles effectively scavenges reactive oxygen species (ROS), reducing oxidative stress markers from ~85% to ~25% at the wound margin.
- The treatment promoted superior re-epithelialization with an epithelial thickness of 80.4 mm and minimal scar length (1.6 mm).
- The fibrous PLGA/Gelatin scaffold provides a biocompatible environment that mimics the extracellular matrix, facilitating cellular migration and tissue integration.
Background: The Challenge of the Infectious Wound Microenvironment
Chronic and infected wounds represent a significant clinical burden, often leading to prolonged hospitalization, increased healthcare costs, and significant patient morbidity. The primary obstacles to effective wound repair in these contexts are multifaceted: persistent bacterial colonization, the formation of resilient biofilms, and a pathologically elevated oxidative microenvironment. Bacterial infections, particularly those involving Staphylococcus aureus (S. aureus), trigger a prolonged inflammatory phase that stalls the transition to the proliferative and remodeling phases of healing.
Concurrently, the overproduction of reactive oxygen species (ROS) exerts severe oxidative stress on surrounding viable tissue, causing cellular damage and hindering the migration of keratinocytes and fibroblasts. Traditional dressings often serve merely as physical barriers and lack the proactive biochemical properties required to modulate this complex microenvironment. Consequently, there is an urgent need for “intelligent” wound dressings that can simultaneously provide antimicrobial protection and antioxidant support to enable scarless skin regeneration.
Study Design and Methodology
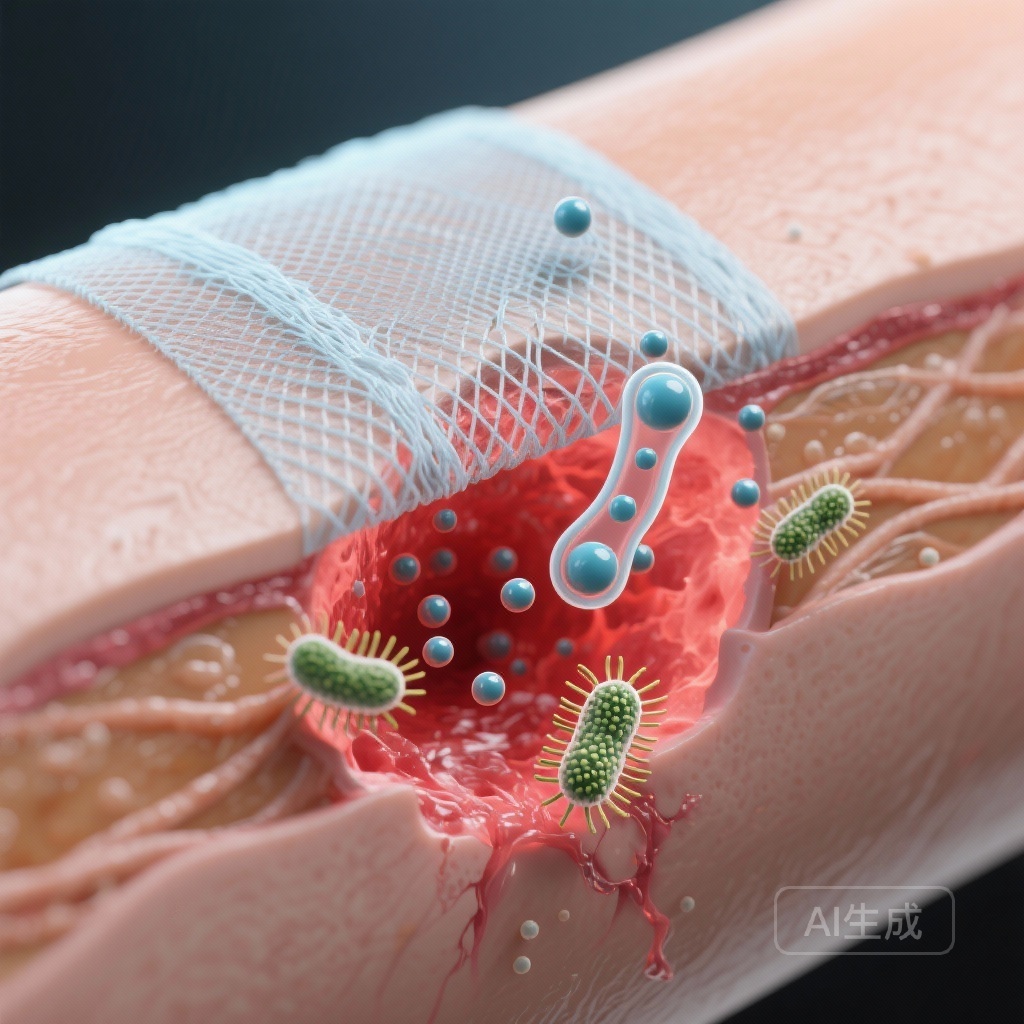
In a study published in Materials Today Bio (2024), researchers engineered a specialized fibrous dressing composed of poly(L-lactide-co-glycolide) and gelatin (PLGA/Gel), integrated with two bioactive agents: immunoglobulin of yolk (IgY) and cerium oxide nanoparticles (CeO2 NPs). The resulting dressing, designated as P/G@IYCe, was designed to leverage the specific strengths of each component.
The Bioactive Components
The immunoglobulin of yolk (IgY) was selected for its potent immunotherapeutic potential. As an alternative to traditional antibiotics, IgY can target antimicrobial-resistant pathogens without contributing to systemic resistance. Cerium oxide nanoparticles were incorporated due to their unique redox-cycling capabilities, which allow them to mimic the activity of superoxide dismutase (SOD) and catalase, thereby neutralizing harmful superoxide radicals.
Experimental Framework
The researchers utilized a rat model featuring full-thickness excisional defects infected with S. aureus to evaluate the in vivo performance of the dressings. The study compared several groups: an untreated control, a plain P/G scaffold (P-control), P/G loaded with IgY only (P/G@IY), P/G loaded with CeO2 only (P/G@Ce), and the synergistic P/G@IYCe dressing. Primary endpoints included wound closure percentage, residual bacterial count, ROS levels, and histological assessments of epithelial thickness and scar formation.
Key Findings: Synergistic Antibacterial and Antioxidant Efficacy
The results of the study demonstrated that the P/G@IYCe dressing significantly outperformed all other groups across nearly every metric of wound healing. By day 14 post-operation, the P/G@IYCe group reached a wound closure percentage of 73.3 ± 3.0%. In stark contrast, the untreated control showed only 16.6 ± 2.9% closure, while the P/G group reached 33.3 ± 1.7%. Even the single-agent dressings, P/G@IY and P/G@Ce, showed substantially lower closure rates than the dual-action formulation, highlighting a clear synergistic effect.
Antimicrobial Performance
The in vivo antimicrobial assays conducted on day 7 revealed that the P/G@IYCe dressing drastically reduced the survival ratio of bacteria at the wound site to approximately 24.6%. Interestingly, the P/G@Ce group also showed significant antibacterial activity (34.2% survival), likely due to the intrinsic antibacterial properties of cerium oxide. The P/G@IY group showed a higher survival rate (118.5%) compared to the control (100%), suggesting that while IgY is effective, its combination with CeO2 is necessary to maximize the antimicrobial microenvironment in this specific scaffold configuration.
Modulating Oxidative Stress
The scavenging of ROS is critical for preventing secondary tissue damage. The researchers found that the P/G@Ce and P/G@IYCe groups exhibited negligible ROS-positive areas during histological analysis. At the wound margin, the ROS-positive cell ratio in the P/G@IYCe group was only 25.7 ± 2.5%, compared to a staggering 89.7 ± 1.9% in the control group. This reduction in oxidative stress directly correlated with a decrease in the number of inflammatory cells observed in H&E staining by day 14, suggesting a faster transition to the regenerative phase of healing.
Histological Insights: Re-epithelialization and Scar Mitigation
A hallmark of successful skin regeneration is the formation of a functional epithelial layer and the minimization of scar tissue. The study’s histological data provided compelling evidence for the regenerative quality of the P/G@IYCe-treated wounds.
Epithelial Regeneration
By day 14, while the control and plain P/G groups lacked an intact epithelial layer, the P/G@IYCe group manifested a robust, intact layer with a thickness of 80.4 ± 11.5 mm. This was significantly thicker than the P/G group (39.8 ± 4.9 mm), indicating that the synergistic bioactive environment promoted rapid keratinocyte proliferation and migration.
Scarless Healing
One of the most clinically relevant findings was the impact on scar length. Excessive scarring can lead to functional impairment and aesthetic concerns for patients. The P/G@IYCe group exhibited a negligible scar length of 1.6 ± 0.4 mm. This was markedly superior to the control group (4.6 ± 0.4 mm) and even the P/G@Ce group (3.0 ± 0.4 mm). The data suggests that the combined reduction in inflammation, bacterial load, and oxidative stress allows the tissue to remodel in a way that closely mimics original skin architecture rather than forming dense fibrotic tissue.
Expert Commentary: A Paradigm Shift in Wound Care
The development of the P/G@IYCe dressing represents a sophisticated shift in biomaterials research—moving from passive scaffolds to active, multi-modal therapeutic platforms. The integration of IgY is particularly noteworthy; as the medical community faces the growing threat of antibiotic resistance, passive immunotherapy via yolk antibodies offers a targeted, non-toxic alternative for managing localized infections.
Furthermore, the use of cerium oxide as a catalytic antioxidant provides a “self-renewing” mechanism for ROS scavenging, which is often more efficient than traditional molecular antioxidants that are consumed during the reaction. The fibrous nature of the PLGA/Gel scaffold provides the necessary topographical cues for cell attachment, essentially creating a “healing factory” at the site of injury. However, clinicians should note that while these results in rat models are promising, the transition to human clinical trials will require careful consideration of the long-term metabolic clearance of cerium oxide and the shelf-life stability of the IgY antibodies within the polymer matrix.
Conclusion
The study by Zhao et al. provides a robust proof-of-concept for the use of IgY and CeO2-loaded PLGA/Gel dressings in treating infectious wounds. By addressing the twin hurdles of bacterial infection and oxidative stress, the P/G@IYCe dressing enables a microenvironment conducive to rapid re-epithelialization and minimal scarring. This research paves the way for a new generation of intelligent dressings that not only protect wounds but actively participate in the complex biological symphony of skin regeneration.
References
Zhao X, Weng C, Feng H, Shafiq M, Wang X, Liu L, Han L, El-Newehy M, Abdulhameed MM, Yuan Z, Mo X, Wang Y. The immunoglobulin of yolk and cerium oxide-based fibrous poly(L-lactide-co-glycolide)/gelatin dressings enable skin regeneration in an infectious wound model. Mater Today Bio. 2024 Dec 17;30:101408. doi: 10.1016/j.mtbio.2024.101408. PMID: 39811611; PMCID: PMC11732107.