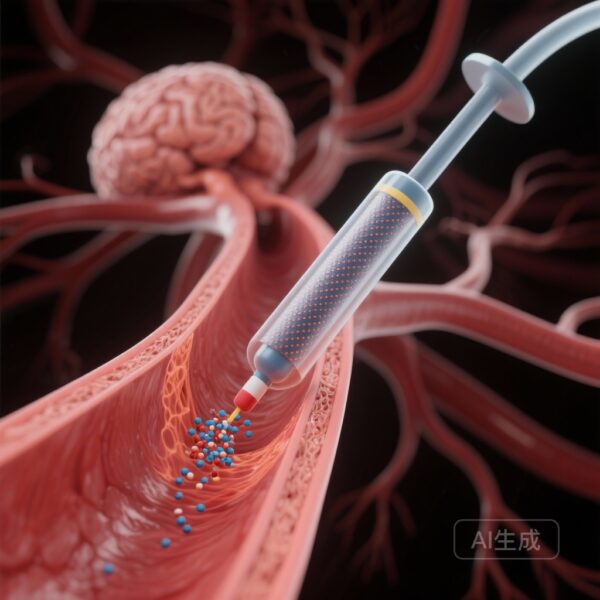
Introduction: The Challenge of Intracranial Restenosis
Symptomatic intracranial atherosclerotic disease (ICAD) remains a leading cause of stroke worldwide, particularly in Asian populations. While medical management is the first line of defense, patients with high-grade stenosis (70-99%) often require endovascular intervention due to a high risk of recurrent ischemic events. Historically, the use of bare-metal stents (BMS) provided a mechanical solution to vessel narrowing, but this approach has been perpetually hampered by high rates of in-stent restenosis (ISR). The inflammatory response triggered by the metallic scaffold often leads to neointimal hyperplasia, effectively re-occluding the vessel over time.
Drug-coated balloons (DCB), which deliver antiproliferative agents like paclitaxel directly to the vessel wall without leaving a permanent foreign body, have emerged as a promising alternative. The DR. BEYOND trial, a multicenter randomized controlled trial recently published in Radiology, provides robust evidence on whether this technology can finally overcome the limitations of traditional stenting in the intracranial circulation.
Highlights of the DR. BEYOND Trial
- DCB angioplasty reduced the risk of 6-month angiographic restenosis by 62% compared to bare-metal stenting.
- Symptomatic restenosis was significantly lower in the DCB group (1% vs 10%).
- Patients treated with DCB experienced a 69% reduction in recurrent ischemic events between 30 days and 1 year post-procedure.
- Periprocedural safety (30-day stroke or death) was comparable between the two interventions.
Study Design and Methodology
The DR. BEYOND study was a prospective, multicenter randomized controlled trial conducted across 14 tertiary hospitals in China. The researchers enrolled 209 participants (median age 59 years) with symptomatic ICAD and high-grade stenosis (70-99%). Participants were randomly assigned in a 1:1 ratio to receive either DCB angioplasty (n=103) or BMS placement (n=106).
The primary efficacy endpoint was the rate of restenosis at 6 months, rigorously assessed via digital subtraction angiography (DSA). Restenosis was defined as >50% stenosis at the treated site or a >20% absolute increase in stenosis for those with residual narrowing. Secondary endpoints included symptomatic restenosis at 6 months and clinical outcomes ranging from 30 days to 1 year, specifically focusing on recurrent ischemic strokes in the target vessel territory.
Key Findings: Efficacy and Safety
Angiographic and Symptomatic Restenosis
The results demonstrated a clear superiority of DCB over BMS in maintaining vessel patency. At the 6-month DSA follow-up, the restenosis rate in the DCB group was 11% (11 of 103), compared to 29% (31 of 106) in the BMS group (risk ratio [RR], 0.38; 95% CI: 0.19, 0.78; P = .006). Perhaps more clinically significant was the reduction in symptomatic restenosis—cases where the narrowing led to renewed neurological deficits—which occurred in only 1% of the DCB group versus 10% of the BMS group (RR, 0.13; 95% CI: 0.02, 0.96; P = .02).
Long-term Clinical Outcomes
The benefits of DCB extended beyond the angiographic findings into long-term clinical protection. Between the 30-day post-procedure mark and the 1-year follow-up, the rate of recurrent ischemic events was significantly lower in the DCB group (4%) compared to the BMS group (13%), representing a hazard ratio (HR) of 0.31 (95% CI: 0.10, 0.94; P = .04). This suggests that preventing early restenosis translates directly into a reduced risk of late-stage strokes.
Safety Profile
Safety is a paramount concern in intracranial interventions, given the fragility of cerebral vessels. The trial found no significant difference in the composite safety endpoint of 30-day stroke or death, which occurred in 6% of the DCB group and 5% of the BMS group (HR, 1.24; 95% CI: 0.38, 4.05; P = .73). This indicates that the use of a drug-coated balloon does not introduce additional acute procedural risks compared to conventional stenting.
Clinical Interpretation and Mechanistic Insights
The success of DCB in this trial can be attributed to the pharmacodynamics of paclitaxel. When the balloon is inflated, the drug is transferred to the arterial wall, where it inhibits the migration and proliferation of smooth muscle cells—the primary drivers of neointimal hyperplasia. Unlike a stent, which remains as a permanent stimulus for inflammation and potential thrombus formation, the DCB leaves nothing behind, allowing the vessel to undergo positive remodeling.
Clinicians should note that while BMS was used as the comparator, the trial addresses a critical gap left by previous studies like SAMMPRIS and VISSIT, which highlighted the high risks of aggressive endovascular therapy. The DR. BEYOND findings suggest that the choice of device is a critical determinant of long-term success. By minimizing the restenosis that often follows mechanical dilation, DCB may offer a more durable solution for high-risk ICAD patients.
Expert Commentary and Limitations
While the results are compelling, some nuances warrant discussion. First, the trial used bare-metal stents as the comparator. In some regions, drug-eluting stents (DES) are also used off-label for ICAD, and future head-to-head trials between DCB and DES would be valuable. Second, the study population was entirely Chinese. Given that the morphology and prevalence of ICAD can vary significantly across ethnicities, further validation in Western populations is necessary to ensure generalizability.
Furthermore, the technical expertise required for DCB—specifically the need for careful pre-dilation to ensure the drug-coated balloon can reach the lesion without losing its coating—means that these results are most applicable in high-volume, tertiary stroke centers. The “leave nothing behind” strategy is elegant, but it requires precise patient selection and procedural execution.
Conclusion
The DR. BEYOND trial establishes drug-coated balloon angioplasty as a superior intervention compared to bare-metal stenting for patients with high-grade symptomatic intracranial stenosis. By drastically reducing both angiographic and symptomatic restenosis, DCBs provide a safer and more effective pathway for secondary stroke prevention in this challenging patient population. As endovascular techniques continue to evolve, the shift toward temporary scaffolds and drug-delivery systems appears to be the most promising direction for improving long-term vascular health in ICAD.
References
1. Guan S, Tong X, Li X, et al. Drug-coated Balloon for Endovascular Treatment of Symptomatic Intracranial Stenotic Disease: A Multicenter Randomized Controlled Trial. Radiology. 2026;318(1):e250893. doi:10.1148/radiol.250893.
2. Chimowitz MI, Lynn MJ, Derdeyn CP, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med. 2011;365(11):993-1003.
3. Zaidat OO, Fitzsimmons BF, Woodward BK, et al. Effect of a balloon-expandable intracranial stent vs medical therapy on risk of stroke in patients with symptomatic intracranial stenosis: the VISSIT randomized clinical trial. JAMA. 2015;313(12):1240-1248.
Funding and Registration
This study was registered with the Chinese Clinical Trial Registry (ChiCTR2100046829). Funding was provided by relevant national health and research grants in China.