Highlight
Dibifree significantly reduces HbA1c, fasting glucose, and postprandial glucose levels when used as an add-on therapy in patients with type 2 diabetes. The intervention successfully lowered body fat percentage and demonstrated sustained metabolic benefits even after a washout period. Mechanistic analysis reveals a multi-target action including the enhancement of GLP-1 secretion, inhibition of DPP-4 and alpha-glucosidase, and promotion of anti-inflammatory M2 macrophage polarization.
Background: The Challenge of Multi-Systemic Metabolic Dysfunction
Type 2 diabetes (T2D) is no longer viewed simply as a disorder of insulin resistance and beta-cell failure; it is recognized as a complex, multi-organ systemic disease involving chronic inflammation, gut dysbiosis, and adipose tissue dysfunction. While pharmacotherapy remains the cornerstone of management, many patients fail to achieve optimal glycemic targets or struggle with the side effects of conventional medications. There is an increasing clinical interest in integrative approaches that utilize functional foods and dietary phytomixes to complement standard care. Dibifree was developed as a standardized formulation of food-derived bioactives specifically designed to address the interconnected pathways of metabolic syndrome. This study evaluates whether this phytomix can provide additive clinical benefits and explores the underlying molecular mechanisms that drive its efficacy.
Study Design: A Rigorous Clinical Evaluation
To evaluate the clinical efficacy of Dibifree, researchers conducted a 7-month randomized, double-blind, placebo-controlled crossover trial. The study enrolled 40 adults diagnosed with T2D. Participants were randomized to receive either Dibifree (15 g/day) or a placebo as an add-on to their existing therapeutic regimen for a three-month period. This was followed by a one-month washout period, after which the groups were switched—those previously on placebo received Dibifree, and vice versa, for another three months. The primary endpoints were changes in HbA1c, fasting plasma glucose (FPG), postprandial glucose (PPG), and body weight. Secondary endpoints included body fat percentage and transcriptomic profiling of cell lines to identify gene expression changes associated with the treatment.
Key Findings: Glycemic Control and Adiposity
The clinical results were robust, showing that Dibifree as an add-on therapy significantly outperformed the placebo across all primary glycemic metrics. Patients treated with the phytomix experienced a significant reduction in HbA1c levels compared to the placebo group. Furthermore, both fasting and postprandial glucose levels were markedly improved. One of the most notable findings was the sustainability of the effect; patients who switched from Dibifree to placebo during the second phase maintained improved glycemic profiles for a period, suggesting a potential ‘carry-over’ or disease-modifying effect on metabolic health.
Beyond glucose management, Dibifree treatment led to a significant decrease in body fat percentage. This suggests that the phytomix does not merely mask hyperglycemia but actively influences lipid metabolism and adiposity. The safety profile was excellent, with no significant adverse events reported, making it a viable long-term adjunctive option for patients requiring better metabolic control.
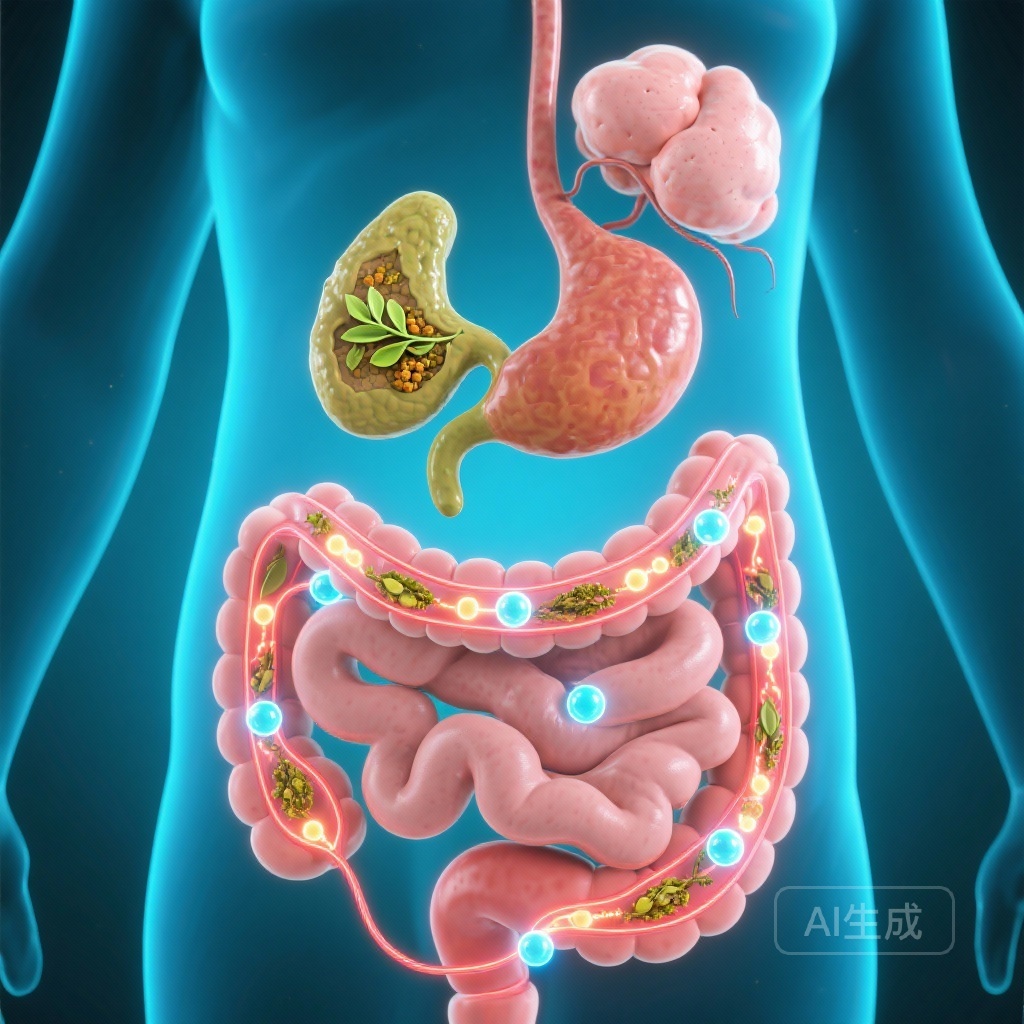
Mechanistic Deep Dive: The Gut-Pancreas-Adipose-Immune Axis
The researchers utilized integrated bioinformatics and functional assays to elucidate how Dibifree achieves these clinical outcomes. The study identified a multi-pronged mechanism of action referred to as the gut-pancreas-adipose-immune axis.
Incretin Modulation and Glucose Metabolism
Transcriptomic profiling revealed that Dibifree treatment enriched pathways related to cAMP signaling and GLP-1 (glucagon-like peptide-1). Functional assays confirmed that the phytomix enhances GLP-1 secretion from intestinal L-cells. Moreover, Dibifree demonstrated the ability to inhibit DPP-4 (dipeptidyl peptidase-4), the enzyme responsible for degrading GLP-1, and alpha-glucosidase, which slows the absorption of carbohydrates in the gut. This dual action mimics aspects of modern incretin-based therapies.
Adipose Tissue and Anti-Glycation Effects
The reduction in adiposity observed in patients was supported by in vitro findings showing that Dibifree suppresses adipogenesis. Additionally, the phytomix was found to reduce the formation of Advanced Glycation End-products (AGEs) and modulate the AGE-RAGE signaling pathway, which is critical in preventing long-term diabetic complications such as nephropathy and cardiovascular disease.
Immune Modulation and M2 Macrophage Polarization
Perhaps the most novel finding was the effect on the immune system. Dibifree promoted the polarization of macrophages toward the M2 phenotype. Unlike M1 macrophages, which are pro-inflammatory and contribute to insulin resistance, M2 macrophages are anti-inflammatory and promote tissue repair and insulin sensitivity. This shift was accompanied by an enrichment of the IL-10 pathway, a key anti-inflammatory cytokine, suggesting that Dibifree helps resolve the chronic low-grade inflammation characteristic of T2D.
Expert Commentary and Clinical Perspectives
The findings from this study are significant because they provide high-level clinical evidence for a functional food intervention in a field often saturated with anecdotal claims. The crossover design adds a layer of statistical power and reliability, as each patient serves as their own control. From a clinical perspective, the ability of Dibifree to target multiple pathways—glucose absorption, incretin secretion, adipocyte differentiation, and immune modulation—offers a holistic advantage over single-target pharmacological agents.
However, clinicians should note that while Dibifree is an effective add-on, it is not a replacement for standard medical therapy. The study’s sample size of 40 participants, while sufficient for a crossover trial, warrants larger multi-center studies to confirm these findings across diverse populations. Furthermore, the 7-month duration provides good mid-term data, but long-term cardiovascular outcome trials would be beneficial to see if these metabolic improvements translate into reduced mortality.
Conclusion
Dibifree represents a promising advancement in the use of dietary phytomixes for type 2 diabetes management. By significantly improving HbA1c and reducing body fat through the modulation of the gut-pancreas-adipose-immune axis, it addresses the underlying pathophysiology of the disease. This study bridges the gap between traditional dietary wisdom and modern evidence-based medicine, providing a scientific rationale for integrating standardized phytomixes into the clinical management of metabolic disorders.
References
Huang TY, Dai NT, Liao HJ, et al. Dibifree, a dietary phytomix, improves glycemic control and adiposity via modulation of the gut-pancreas-adipose-immune axis in type 2 diabetes. Food Res Int. 2026 Jan;223(Pt 1):117820. doi: 10.1016/j.foodres.2025.117820. Epub 2025 Nov 13. PMID: 41352784.
American Diabetes Association. Standards of Care in Diabetes—2024. Diabetes Care. 2024;47(Supplement 1).