Highlights
- In a Phase 2 trial (ADVL1521), denosumab failed to meet primary efficacy endpoints in both measurable and resected recurrent osteosarcoma cohorts.
- No objective radiographic responses (RECIST) were observed among patients with measurable disease.
- While pharmacodynamic markers (CTx and NTx) confirmed target engagement, this did not translate into clinical benefit or improved event-free survival.
- The safety profile was consistent with previous denosumab studies, with hypophosphatemia and hypocalcemia being the most frequent high-grade adverse events.
Background: The Challenge of Recurrent Osteosarcoma
Osteosarcoma remains the most common primary malignant bone tumor in children and adolescents. While frontline therapy involving surgery and multi-agent chemotherapy (MAP: methotrexate, doxorubicin, and cisplatin) has significantly improved survival for localized disease, the outlook for patients with recurrent or refractory osteosarcoma remains dismal. Long-term survival for those with relapsed disease is typically less than 20%, a statistic that has remained largely stagnant for decades. This unmet medical need has driven the search for targeted therapies that can disrupt the tumor microenvironment or specific signaling pathways essential for osteosarcoma progression.
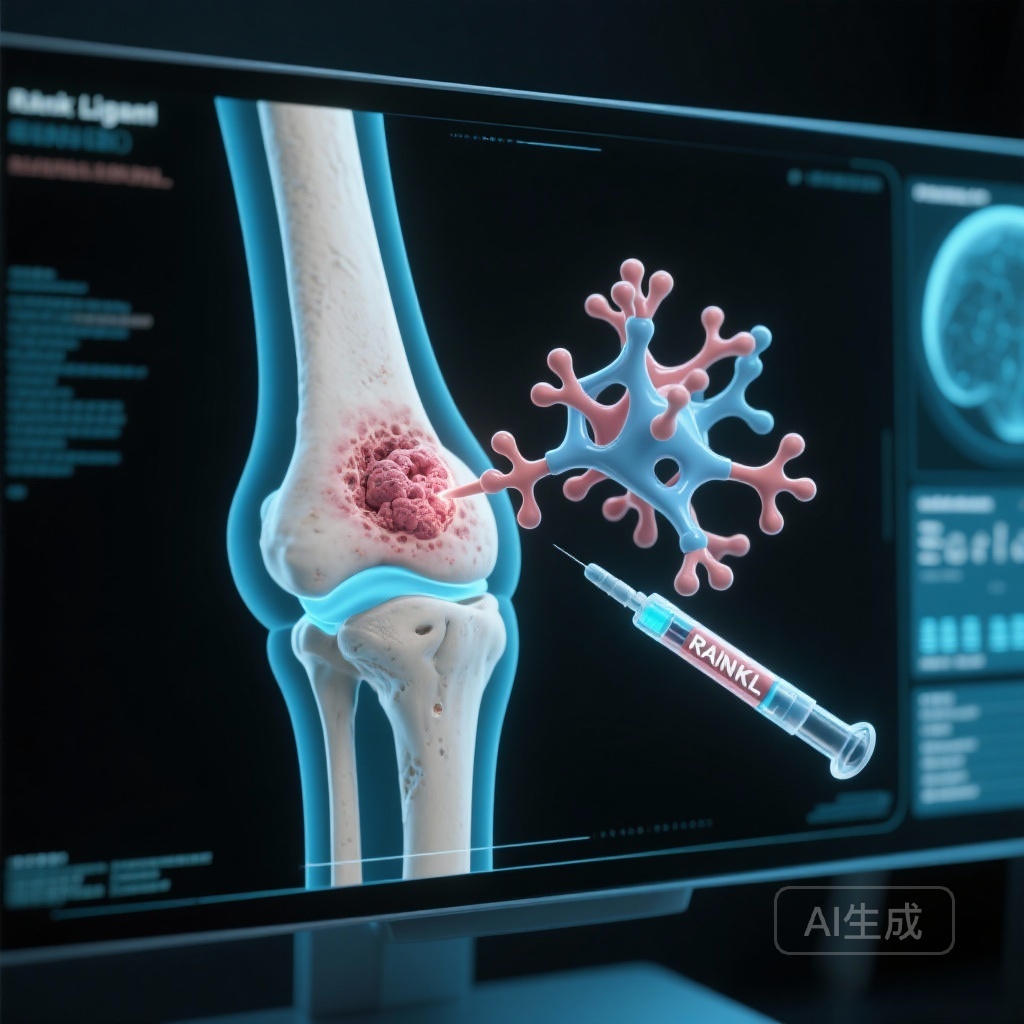
One such pathway involves the Receptor Activator of Nuclear Factor-kappa B (RANK) and its ligand (RANKL). RANKL is a critical mediator of osteoclast formation, function, and survival. In the context of bone malignancies, the “vicious cycle” of bone destruction suggests that tumor cells stimulate osteoclasts to resorb bone, which in turn releases growth factors that promote further tumor expansion. Preclinical studies using mouse models of osteosarcoma suggested that RANKL inhibition could reduce bone destruction, limit tumor growth, and potentially inhibit pulmonary metastasis. Denosumab, a fully human monoclonal antibody against RANKL, is already FDA-approved for giant cell tumor of bone and bone metastases from solid tumors, making it a logical candidate for clinical evaluation in osteosarcoma.
Study Design and Methodology
The Children’s Oncology Group (COG) conducted this single-arm, open-label phase 2 trial to evaluate the efficacy and safety of denosumab in patients with recurrent or refractory osteosarcoma. The study enrolled skeletally mature patients aged 11 to 49 years across two distinct cohorts:
Cohort 1: Measurable Disease
This cohort included patients with measurable disease by RECIST 1.1 criteria. The primary objectives were to assess objective response rate and the proportion of patients remaining event-free at 4 months. Historically, a 4-month event-free survival (EFS) rate of approximately 20% has been the benchmark for activity in this population.
Cohort 2: Completely Resected Disease
This cohort consisted of patients who had achieved a complete surgical resection of all known sites of disease. The primary objective was the 12-month event-free survival rate, with a benchmark derived from historic data where 12-month EFS in similar patients was approximately 20% to 25%.
Intervention
All participants received denosumab at a dose of 120 mg administered subcutaneously every 4 weeks. To mitigate the risk of hypocalcemia, patients were required to take calcium and vitamin D supplementation. Secondary endpoints included toxicity monitoring, pharmacokinetics (PK), and pharmacodynamic (PD) effects measured via serum and urine markers of bone turnover.
Key Findings: Efficacy and Safety
The trial enrolled a total of 15 evaluable patients in Cohort 1 and 38 evaluable patients in Cohort 2. Unfortunately, the results indicated that denosumab did not achieve the predefined activity levels required for further development in this setting.
Clinical Efficacy
In Cohort 1 (measurable disease), there were zero objective responses. Only one out of the 15 patients remained event-free at the 4-month mark. In Cohort 2 (resected disease), 10 out of 38 patients remained event-free at 12 months. Both cohorts failed to meet the statistical thresholds for success. The median EFS for the entire study population was disappointingly brief, reinforcing the aggressive nature of recurrent osteosarcoma.
Pharmacokinetics and Pharmacodynamics
Despite the lack of clinical efficacy, the study confirmed that denosumab was biologically active. Pharmacokinetic analysis showed mean serum trough concentrations at steady state (cycles 2 through 7) ranging from 23.7 to 31 μg/mL, which is consistent with therapeutic levels observed in other indications. Pharmacodynamic markers demonstrated the expected suppression of bone resorption; serum c-telopeptide (CTx) and urine n-telopeptide (NTx) levels decreased significantly during treatment, indicating successful RANKL inhibition and subsequent reduction in osteoclast activity.
Safety and Toxicity
Denosumab was generally well-tolerated. The most common Grade 3 or higher adverse events were electrolyte disturbances: hypophosphatemia (11%) and hypocalcemia (8%). These findings are consistent with the known mechanism of denosumab, which prevents the release of calcium and phosphorus from the bone matrix into the bloodstream. No cases of osteonecrosis of the jaw (ONJ) or atypical femoral fractures—rare but serious side effects associated with long-term denosumab use—were reported in this cohort, though the duration of exposure was relatively short for most patients.
Expert Commentary: Why Did Denosumab Fail?
The failure of denosumab in this trial, despite strong preclinical evidence, highlights the complexity of translating mouse model findings to human clinical practice in osteosarcoma. Several factors may explain these results. First, while RANKL inhibition effectively stops osteoclast-mediated bone resorption, it may not have a direct cytotoxic effect on the osteosarcoma cells themselves. In many cases of osteosarcoma, the tumor cells may be less dependent on the bone-remodeling “vicious cycle” than initially hypothesized, especially in the setting of advanced or metastatic disease where pulmonary lesions (which are not in bone) predominate.
Furthermore, osteosarcoma is characterized by extreme genomic instability and intratumoral heterogeneity. Targeting a single pathway like RANK/RANKL may be insufficient to counteract the multiple redundant growth signals present in these tumors. The historic benchmarks used by the COG are also rigorous; for a drug to be considered successful, it must show a clear signal above the background of very poor outcomes in this patient population.
It is also worth noting that the PD markers confirmed the drug was doing exactly what it was designed to do—inhibit bone turnover. The fact that the tumor continued to progress despite this inhibition suggests that RANKL is not a primary driver of tumor growth in the majority of recurrent osteosarcoma cases.
Conclusion
The COG Phase 2 trial of denosumab in recurrent/refractory osteosarcoma provides a clear, albeit negative, answer regarding the utility of single-agent RANKL inhibition in this setting. While denosumab demonstrated a manageable safety profile and expected pharmacokinetic and pharmacodynamic effects, it lacked the clinical activity necessary to justify further clinical development for this specific indication. These results emphasize the ongoing need for novel therapeutic strategies and perhaps more sophisticated preclinical models that better reflect the biological complexity of human osteosarcoma. Future research may focus on combining bone-targeted agents with immunotherapy or newer tyrosine kinase inhibitors to achieve more durable responses.
Funding and ClinicalTrials.gov
This study was supported by the National Cancer Institute (NCI) and the Children’s Oncology Group. ClinicalTrials.gov Identifier: NCT02470091 (ADVL1521).
References
- Janeway KA, Chou AJ, Buxton A, et al. A Phase 2 Trial of RANKL Antibody, Denosumab, in Two Cohorts of Patients with Recurrent/Refractory Osteosarcoma, a Report from the Children’s Oncology Group. Clin Cancer Res. 2026;32(1):36-44. doi:10.1158/1078-0432.CCR-24-2885.
- Gorlick R, Janeway K, Khanna C, et al. Osteosarcoma: Progress through collaboration. J Clin Oncol. 2021;39(16):1711-1725.
- Lancia C, et al. The RANK/RANKL pathway in bone oncology. Frontiers in Bioscience. 2022;27(4):122.