Highlights
– In 51 patients with severe single‑vessel coronary disease, greater collateral flow was strongly associated with more physiological ischemia (lower FFR/iFR) but with lower experimentally provoked chest pain intensity.
– Daily angina frequency showed little correlation with invasive physiologic indices (FFR, iFR), suggesting collaterals help explain the commonly observed mismatch between stenosis severity and symptoms.
– Sequential brief balloon occlusions did not produce measurable ischemic preconditioning or progressive collateral recruitment, arguing that collateral protection was a stable trait in the acute setting.
Background
Clinicians frequently encounter patients in whom the anatomic severity of coronary stenosis, physiological measures of ischemia, and symptom burden diverge. Some patients with significant physiological ischemia report minimal angina, whereas others with less apparent ischemia experience disabling chest pain. Coronary collateral vessels—pre‑existing or recruited channels connecting epicardial arteries—have long been proposed as a protective mechanism that can limit ischemia and buffer symptom expression. However, direct human experimental evidence linking collateral flow to the intensity of ischemic chest pain has been limited.
Study design
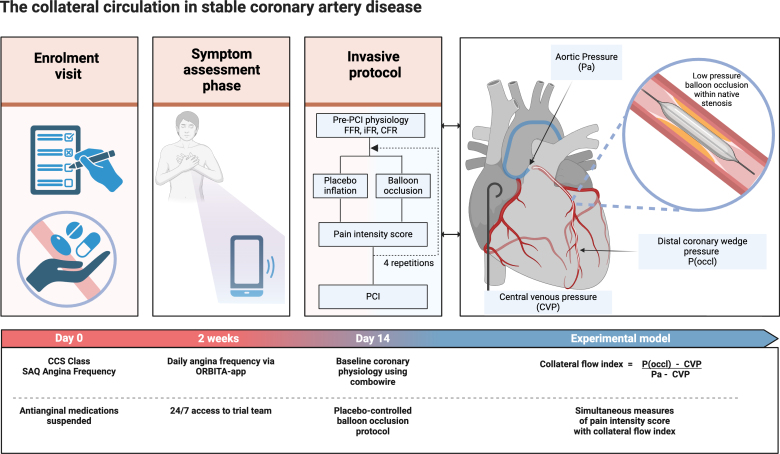
The report by Rajkumar et al. (Circulation 2025) presents a prospective, placebo‑controlled, invasive n‑of‑1 series in 51 adults with severe single‑vessel coronary artery disease and angina (NCT04280575). Key design elements:
- Antianginal medications were stopped to avoid symptom suppression, and daily angina frequency was recorded with a dedicated smartphone app for 14 days before invasive testing (the app used in ORBITA was adapted for symptom capture).
- Baseline physiologic assessment included fractional flow reserve (FFR), instantaneous wave‑free ratio (iFR), and coronary flow reserve (CFR).
- Each participant underwent four 60‑second low‑pressure balloon occlusions across the culprit stenosis. Each true occlusion was paired with an audiovisually identical sham (placebo) inflation in randomized order and blinded to the participant, forming an n‑of‑1 placebo‑controlled experiment within each patient.
- After each episode (real or sham), participants rated pain intensity on a 0–10 numeric scale; a placebo‑controlled pain score (subtracting sham response) provided the primary symptom measure.
- Collateral flow index (CFI) was calculated from simultaneous aortic, right atrial and distal coronary wedge pressures during balloon occlusion — an established invasive measure of collateral contribution to distal perfusion.
- Associations were assessed with Bayesian models and Somers’ D rank correlations; reported Pr values indicate the posterior probability favoring the association.
Key findings
Population characteristics and baseline physiology
The cohort had a mean age of 63±9 years; 78% were men. Median FFR was 0.68 (IQR 0.57–0.79), median iFR 0.80 (IQR 0.48–0.89), and median CFR 1.42 (IQR 1.08–1.85), indicating that many participants had physiologically significant stenoses despite variable flow reserve.
Relationship between daily angina frequency and physiologic ischemia
Daily angina frequency recorded over 14 days showed little correlation with physiological indices: Somers’ D for FFR vs angina frequency was 0.124 (Pr = 0.057) and for iFR vs angina frequency 0.056 (Pr = 0.150). In other words, more severe physiological ischemia did not predict a higher day‑to‑day angina burden in this cohort.
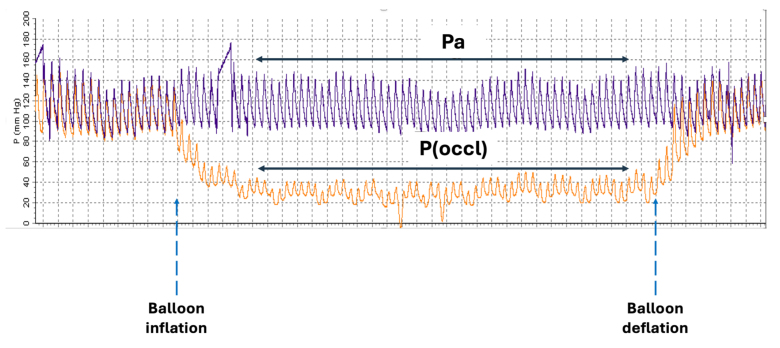
Collateral flow correlates with ischemic burden
There was strong evidence that lower FFR and iFR values (greater ischemic burden) were associated with higher collateral flow (Somers’ D 0.302, Pr = 0.998 for FFR; Somers’ D 0.316, Pr = 0.999 for iFR). This indicates that more severe physiologic stenoses tend to have greater collateral recruitment.
Collateral flow associates with lower experimentally provoked pain intensity
Crucially, higher CFI correlated with lower pain intensity scores during the controlled balloon occlusions (Somers’ D 0.341, Pr = 0.999). In practical terms, patients with better collateral circulation experienced less chest pain during identical periods of controlled ischemia, suggesting collateral vessels buffer ischemic nociception.
Stability across sequential occlusions — limited evidence for preconditioning
Within individual patients, pain intensity scores and CFI values remained stable between sequential balloon occlusion episodes, providing little evidence that short repeated occlusions produced progressive collateral recruitment or an ischemic preconditioning effect detectable by pain or CFI in this experimental setup.
Interpretation of effect size and clinical meaning
Somers’ D values in the range ≈0.30 represent moderate associations. The Bayesian Pr values approaching 1.0 provide high posterior confidence in the observed relationships within this cohort, although they do not by themselves establish causality beyond the within‑patient experimental context. Importantly, the placebo‑controlled n‑of‑1 design strengthens the inference that the measured differences in pain reflect true physiological attenuation by collaterals rather than expectation or other nonspecific factors.
Expert commentary and mechanistic insights
Physiologic plausibility
Collateral vessels can maintain distal perfusion, reduce the magnitude of regional ischemia, and therefore reduce activation of ischemia‑sensitive myocardial nociceptors. The present study operationalized collateral function invasively (CFI) and directly measured subjective ischemic chest pain during standardized coronary occlusion with randomized sham controls, providing arguably the clearest evidence to date in humans that collateralization attenuates ischemic pain intensity.
Implications for the ischemia‑symptom disconnect
The finding that daily angina frequency did not correlate well with FFR or iFR but that CFI linked both to ischemic burden and to lower pain intensity suggests that collaterals are an important mediator of the nonlinearity observed between stenosis, ischemia, and angina. Patients with substantial collaterals may tolerate larger ischemic burdens with less symptomatic expression, which could influence clinical decisions about revascularization when based on symptoms alone.
No measurable ischemic preconditioning in short sequential occlusions
Ischemic preconditioning refers to reduced ischemic injury or symptoms after brief prior ischemia and has been demonstrated in experimental settings. The absence of detectable preconditioning in this study may reflect the short duration (60 s) of occlusions, the inter‑episode timing, the dominant role of anatomical collaterals that are relatively fixed in the acute timeframe, or insufficient sample size to detect small within‑patient changes.
Limitations and generalizability
- Sample size and population: 51 patients with severe single‑vessel disease, predominantly male, limit generalizability to women and to multivessel disease.
- Setting of collaterals measurement: CFI measured during controlled balloon occlusion may not precisely reflect resting collateral support in chronic everyday life.
- Stopping antianginals: Withdrawal of antianginal therapy strengthens symptom measurement during testing but may alter baseline physiology and collateral dynamics relative to usual care.
- Subjective pain measure: Although the placebo‑controlled sham design reduces bias, pain ratings remain subjective and influenced by individual pain perception and psychological factors.
- Single‑center or limited centers: The paper reports a carefully controlled invasive protocol; multi‑center replication would bolster external validity.
Clinical implications
These findings help clinicians understand why some patients with physiologically significant lesions report little angina: collaterals can blunt ischemic nociception. When deciding on revascularization, it is important to integrate an anatomic and physiologic assessment with an appreciation of collateral function and patient‑reported symptom burden. The data do not imply that collaterals eliminate the ischemic risk of adverse events; they primarily address symptom modulation. Moreover, the presence of robust collaterals in the setting of significant ischemia could influence the perceived urgency or symptomatic benefit expected from revascularization, but decisions must still account for objective ischemic risk, viability, and prognostic evidence.
Future directions
Key unanswered questions include whether therapies can reliably augment collateral development or function in humans, whether collateral presence modifies long‑term outcomes independently of ischemia and revascularization, and how collateral assessment might be integrated into routine decision‑making. Larger, diverse cohorts and longitudinal studies linking CFI with hard cardiovascular outcomes would help clarify the prognostic significance of collaterals beyond symptom modulation.
Conclusion
The invasive, placebo‑controlled n‑of‑1 study by Rajkumar et al. provides compelling human evidence that coronary collateral circulation is associated with increased physiologic ischemia yet attenuates ischemic chest pain intensity during controlled occlusion. These observations offer a plausible mechanism for the frequent clinical discordance between stenosis severity, ischemia, and angina symptoms and emphasize the importance of considering collateral function in evaluating symptomatic stable coronary disease.
Funding and registration
Clinical trial registration: https://www.clinicaltrials.gov; Identifier: NCT04280575.
Key reference
Rajkumar CA, Foley MJ, Ahmed‑Jushuf F, et al. The Role of the Collateral Circulation in Stable Angina: An Invasive Placebo‑Controlled Study. Circulation. 2025 Dec 2;152(22):1541‑1551. doi:10.1161/CIRCULATIONAHA.125.074687 IF: 38.6 Q1 . PMID: 41144984 IF: 38.6 Q1 ; PMCID: PMC12655870 IF: 38.6 Q1 .
Selected contextual references
1. Al‑Lamee R, Thompson D, Dehbi H‑M, et al. Percutaneous coronary intervention in stable angina (ORBITA): a double‑blind, randomised controlled trial. Lancet. 2018;391(10115):31‑40.
2. Tonino PA, De Bruyne B, Pijls NH, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360(3):213‑224.
3. Davies JE, Sen S, De Bruyne B, et al. Use of the instantaneous wave‑free ratio or fractional flow reserve in PCI. N Engl J Med. 2017;376(19):1824‑1834. (DEFINE‑FLAIR and iFR‑SWEDEHEART trials reported in 2017 collectively assessing iFR vs FFR).
4. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41(3):407‑477.
5. Rentrop KP, Cohen M, Blanke H, Phillips RA. Changes in collateral channel filling immediately after controlled coronary artery occlusion by angioplasty. J Am Coll Cardiol. 1985;5(3):587‑592.
Author note
This summary is written for clinicians and physician‑scientists to interpret the clinical and mechanistic implications of the trial. Clinicians should integrate these findings with guideline recommendations and individual patient context when planning investigations or revascularization strategies.