Highlights
– The multicenter external validation of the IMPACT tool (n=1248) demonstrated good discrimination (C-statistic 0.80) and calibration (Brier score 0.12) for predicting progression of incidental meningioma.
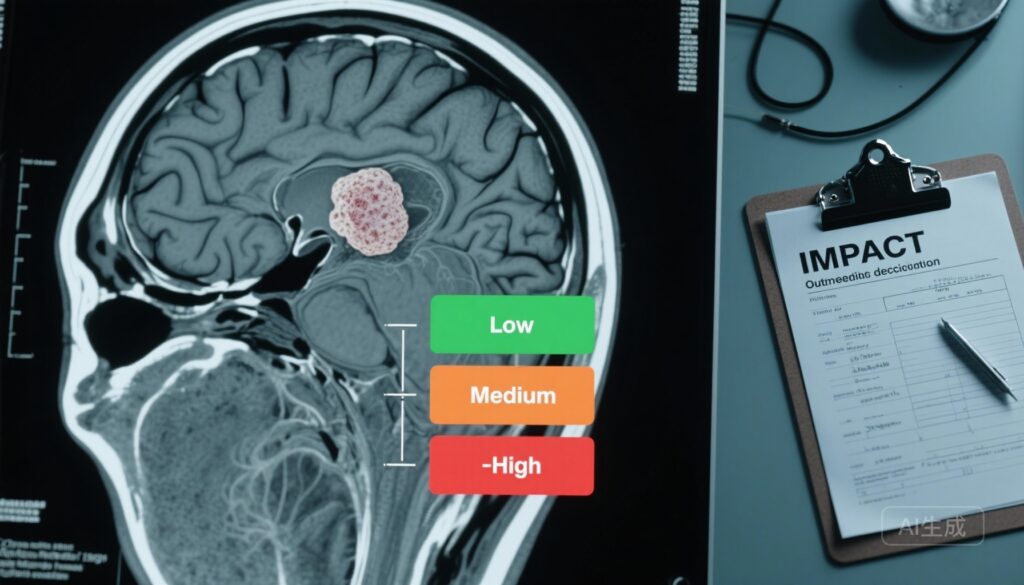
– Five- and 10-year progression-free survival were high (88.1% and 85.7%); risk strata conferred markedly different progression probabilities (low 3.9%, medium 24.2%, high 51.6%).
– Patients with high comorbidity (age-adjusted Charlson Comorbidity Index ≥6) and poor performance status (ECOG 2–4) were more likely to die of other causes than to undergo meningioma-directed intervention.
Background and clinical need
Meningiomas are the commonest primary intracranial tumour in adults and are frequently discovered incidentally because of the widespread use of brain MRI. Most incidentally detected meningiomas follow an indolent course, but a minority progress to growth, cause symptoms, or reach a stage when curative treatment is no longer feasible. Current outpatient practice varies widely: some centres favour early surgery or radiosurgery, others adopt serial imaging with long follow-up, and some patients may be safely discharged. A reliable, externally validated clinical tool that integrates patient-level variables and imaging features to stratify risk would help standardize care, avoid unnecessary surveillance, and identify patients who would benefit from early intervention.
Study design and population
Islim and colleagues report a retrospective, international, multicentre external validation of the IMPACT (Incidental Meningioma: Prognostic Analysis Using Patient Comorbidity and Magnetic Resonance Imaging Tests) clinical prediction tool. The study included 33 centres across 15 countries and captured adults diagnosed with an incidental meningioma between January 2009 and December 2010. Patients were followed until meningioma-directed intervention, tumour progression, death, or last clinical encounter; data collection closed on December 31, 2023. Exclusion criteria included radiation-induced meningioma and NF2-related schwannomatosis. Statistical analyses were conducted after follow-up completion, with model performance assessed by discrimination, calibration, and decision-relevant outcomes.
Primary and secondary endpoints
The primary outcome was a composite endpoint comprising tumour growth, development of meningioma-related symptoms, meningioma-related mortality, and endpoints indicating loss of a window for curative treatment (for example, progression beyond safe resection or radiosurgery). Secondary outcomes included the occurrence of meningioma-directed intervention (surgery or stereotactic radiosurgery) and non-meningioma-related mortality.
Key findings
Population and follow-up: The validation cohort comprised 1248 patients with a median age of 66 years (IQR 55–77); 80% were female (n=999). In total, 945 patients (75.7%) had treatment‑naive meningiomas at the time of diagnosis. Median follow-up was 61 months (IQR 17–108), yielding long-term outcome data in many patients.
Events and survival: During follow-up, 114 tumours (11.3%) in 113 patients (12.0%) met the composite progression endpoint. A total of 132 tumours (13.1%) in 126 patients (13.3%) underwent meningioma-directed intervention. Notably, 383 patients (40.5%) died of causes unrelated to their meningioma without prior tumour progression or intervention.
Progression‑free survival: The estimated progression-free survival (PFS) was 88.1% (95% CI 85.8%–90.5%) at 5 years and 85.7% (95% CI 83.2%–88.2%) at 10 years, emphasizing that most incidental meningiomas remain quiescent over clinically relevant intervals.
Risk stratification performance: Application of the IMPACT tool yielded clear separation between prognostic groups. The observed progression risks were 3.9% for low‑risk meningiomas, 24.2% for medium‑risk, and 51.6% for high‑risk (χ2 P < .001). Model performance metrics supported adequate external validity (Brier score = 0.12; overall C‑statistic = 0.80; 10‑year AUC = 0.83), indicating good discrimination between those who did and did not experience the composite endpoint.
Competing risk insights: The investigators highlighted that patients with heavy comorbidity burden (age‑adjusted Charlson Comorbidity Index [ACCI] ≥6 — for example, an 80‑year‑old with diabetes and prior myocardial infarction) and poor performance status (ECOG 2–4) were more likely to die from other causes than to receive meningioma treatment. This finding supports tailoring follow‑up intensity by life expectancy and functional status.
Model robustness: The study reports that adding additional variables in Cox regression did not materially change the IMPACT tool’s statistical significance, suggesting the tool is not easily confounded by routine clinical covariates in this diverse cohort.
Interpretation and clinical relevance
The IMPACT tool, externally validated in a large, geographically diverse cohort with long follow‑up, appears capable of identifying patients at very low risk of clinically relevant meningioma progression (low‑risk group) who might be safely discharged or subjected to less intensive surveillance. Conversely, patients in the high‑risk stratum face a substantial (>50%) risk of progression over the follow‑up interval and may benefit from early multidisciplinary discussion regarding surgery or radiosurgery.
Importantly, the data underscore the need to contextualize meningioma risk within the patient’s overall health. In older patients with high ACCI and poor functional status, the competing risk of death from other causes often outweighs the risk from an incidental meningioma, arguing for conservative approaches or discharge with safety‑netting in selected cases.
Practical application: integrating the IMPACT tool into outpatient pathways
While the full statistical specification of the IMPACT algorithm is detailed in the primary manuscript, the practical implications for outpatient management are straightforward:
- Low‑risk patients: very low progression probability (≈4%) — consider discharge or minimal imaging (single confirmatory scan then symptom‑triggered review), especially when life expectancy is limited.
- Medium‑risk patients: appreciable progression risk (~24%) — schedule structured serial MRI surveillance with predefined triggers for multidisciplinary review (growth thresholds, new symptoms, or radiological features indicating aggressive behaviour).
- High‑risk patients: high progression probability (>50%) — discuss early definitive treatment options (surgery or stereotactic radiosurgery) in multidisciplinary team settings, balanced against patient comorbidity and preferences.
These broad pathways should be adapted locally and embedded within shared decision‑making conversations that include life expectancy, performance status, tumour location (eloquent cortex, skull base, venous sinus involvement), and patient values.
Strengths and limitations
Strengths: This study’s multicenter design, large sample size, long follow‑up, and focus on an international cohort improve generalizability. External validation—rather than derivation alone—adds confidence that the IMPACT tool retains performance across different practice settings. The explicit attention to competing risks (nonmeningioma mortality) reflects pragmatic clinical decision contexts.
Limitations: Retrospective data collection may introduce selection and information bias, and imaging protocols and reporting standards likely varied between centres in 2009–2010-era scans. The study excluded radiation‑induced and NF2‑related meningiomas, so findings may not generalize to these groups. The report focuses on aggregate tool performance; local validation and prospective implementation studies will be important to confirm real‑world utility, cost‑effectiveness, and impact on patient‑centred outcomes.
Expert commentary and guideline context
Contemporary guidelines emphasize individualized management of incidental meningiomas, balancing tumour biology, location, patient comorbidity, and preferences. A validated prognostic tool that is feasible in outpatient settings can reduce practice variation and focus resources on patients most likely to benefit from intervention. The IMPACT tool adds to a small but growing toolkit for evidence‑based triage of incidental intracranial tumours.
Research and implementation priorities
Key next steps include prospective implementation studies that assess whether using IMPACT changes clinician behaviour, reduces unnecessary imaging or delays needed treatment, and affects patient anxiety and quality of life. Health economic analyses will help determine whether risk‑stratified follow‑up reduces costs without compromising outcomes. Finally, integrating IMPACT into electronic medical records or neuro‑oncology clinic workflows (with automated calculation from clinical and radiology inputs) would facilitate routine use, but requires careful validation in local patient populations and imaging environments.
Conclusions
The IMPACT tool demonstrates adequate external validity for predicting clinically meaningful outcomes among patients with incidental meningioma in this international cohort. Its risk strata differentiate patients at very low, intermediate, and high risk of progression, providing a rational framework to guide outpatient management decisions — from safe discharge to serial imaging or timely intervention. Clinicians should interpret IMPACT scores alongside patient comorbidity, performance status, and preferences, and prospective studies are warranted to confirm benefits of implementation.
Funding and registration
Funding sources, conflicts of interest, and trial or registry identifiers are reported in the primary JAMA Oncology article by Islim et al. Readers should consult the original publication for detailed funding and governance statements.
Selected references
1. Islim AI, Millward CP, Zakaria R, et al; IMPACT Study Investigators, International Consortium on Meningioma (ICOM) and British Neurosurgical Trainee Research Collaborative (BNTRC). A Clinical Tool to Identify Incidental Meningioma for Early Outpatient Management. JAMA Oncol. 2025 Nov 20:e254821. doi:10.1001/jamaoncol.2025.4821. Epub ahead of print. PMID: 41264316; PMCID: PMC12635927.
2. Wiemels J, Wrensch M, Claus EB. Epidemiology and etiology of meningioma. J Neurooncol. 2010 Aug;99(3):307-314. doi:10.1007/s11060-010-0386-3.
AI thumbnail prompt
A high-resolution axial brain MRI slice showing a small, clearly demarcated extra‑axial convexity meningioma at the right frontal convexity. To the left, a clinician’s clipboard with a sheet titled ‘IMPACT’ and three colored risk bars (green, amber, red). Soft clinical lighting, neutral hospital consultation room background, realistic medical imaging detail, subtle overlay of faint data grid for a modern, professional tone.