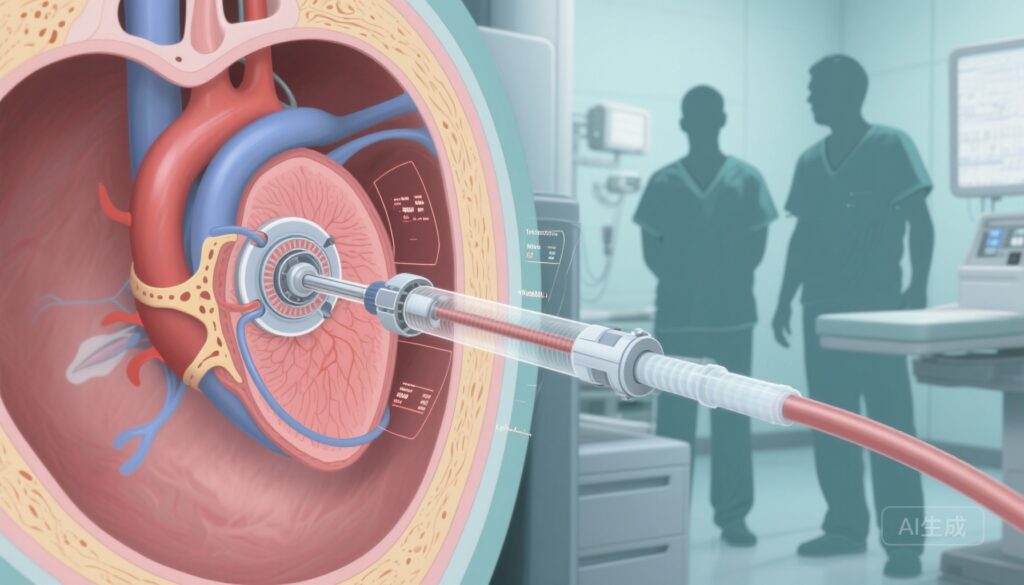
Highlights
– In a prospective, multicentre single‑arm pivotal trial (ENCIRCLE; Lancet 2025), percutaneous transseptal TMVR with the SAPIEN M3 achieved a 1‑year composite of all‑cause mortality or heart‑failure rehospitalisation of 25.2% (95% CI 20.6–30.6), well below the prespecified performance goal of 45% (p<0.0001).
– Procedures were performed without intraprocedural deaths, conversions to surgery, or instances of left ventricular outflow tract (LVOT) obstruction causing haemodynamic compromise; follow‑up completeness was 81% at 1 year.
– The findings support a therapeutic option for a high‑risk group of patients with symptomatic moderate‑to‑severe or severe mitral regurgitation (MR) who are not candidates for surgery or transcatheter edge‑to‑edge repair (TEER), but confirmatory randomised data and longer‑term durability data remain necessary.
Background and Unmet Clinical Need
Mitral regurgitation is a common valve disorder associated with progressive symptoms, heart failure, and increased mortality. A substantial proportion of patients with severe MR are denied surgical mitral valve repair or replacement because of frailty, comorbidities, or prohibitive surgical risk. For many, transcatheter edge‑to‑edge repair (TEER; e.g., MitraClip) offers an alternative, but anatomic constraints (large coaptation gaps, leaflet pathology, calcification, or annular dilation) and suboptimal outcomes in some phenotypes mean not all patients are suitable for TEER. This therapeutic gap has catalysed development of transcatheter mitral valve replacement (TMVR) devices intended to provide valve replacement via percutaneous access for patients otherwise without durable options.
Study Design
The ENCIRCLE trial (NCT04153292) is a prospective, multicentre, single‑arm, pivotal study enrolling adult patients (≥18 years) with symptomatic moderate‑to‑severe or severe MR judged unsuitable for mitral surgery and TEER by a multidisciplinary Heart Team. Recruitment occurred at 56 centres across six countries (USA, Canada, UK, Netherlands, Israel, and Australia). Enrolled patients underwent percutaneous, transseptal implantation of the Edwards SAPIEN M3 TMVR system.
The primary endpoint was a non‑hierarchical composite of all‑cause mortality and heart‑failure rehospitalisation at 1 year in the as‑treated population, compared against a prespecified performance goal of 45%. Key safety and procedural endpoints included intraprocedural mortality, LVOT obstruction causing haemodynamic compromise, and conversion to open surgery. The sponsor was Edwards Lifesciences; the trial was reported as ongoing at the time of publication.
Key Findings
Population and Procedural Characteristics
Between June 9, 2020, and Oct 10, 2023, 1,171 patients were screened and 299 underwent attempted TMVR with the SAPIEN M3. Median age was 77.0 years (IQR 70.0–82.0); sex distribution was approximately equal (51% male, 49% female). The mean Society of Thoracic Surgeons (STS) predicted risk of 30‑day mortality for mitral valve replacement was 6.6%, indicating a high‑risk cohort. Median follow‑up was 1.4 years (IQR 1.0–2.1); clinical follow‑up was available in 283 (95%) patients at 30 days and 243 (81%) at 1 year.
Primary Outcome
The as‑treated 1‑year rate of the composite primary endpoint (all‑cause mortality or heart‑failure rehospitalisation) was 25.2% (95% CI 20.6–30.6). This rate was significantly lower than the prespecified performance goal of 45% (p<0.0001). The trial report presents this result as meeting the primary effectiveness objective.
Periprocedural Safety and Key Secondary Observations
Notable procedural safety findings included no intraprocedural deaths, no conversions to open surgery, and no documented instances of LVOT obstruction that produced haemodynamic compromise. The manuscript states that TMVR with the SAPIEN M3 effectively reduced mitral regurgitation with low rates of complications and mortality, though granular data on the degree of residual MR, detailed breakdown of mortality versus rehospitalisation components, stroke, bleeding, conduction disturbances, valve thrombosis, and need for long‑term anticoagulation are summarized in the primary manuscript and supplemental tables.
Follow‑up Completeness and Data Limitations
Clinical follow‑up at 1 year was available for 81% of treated patients. As with many single‑arm device studies, loss to follow‑up and the absence of a randomized comparator group complicate causal inference. The trial used a prespecified performance goal (45%) rather than a contemporaneous control arm; while historical controls and performance goals are commonplace in device development, they carry inherent limitations versus randomised comparisons.
Interpretation and Clinical Implications
The ENCIRCLE pivotal trial indicates that transseptal TMVR with the SAPIEN M3 can be performed safely in a high‑risk population traditionally excluded from surgery or TEER, and that the procedure is associated with clinically meaningful reductions in the composite of death and heart‑failure readmission at 1 year compared with a conservative performance benchmark. The absence of intraprocedural deaths, conversions to surgery, or haemodynamically significant LVOT obstruction are important safety signals for a transseptal TMVR technology, because previous experience with TMVR (particularly transapical approaches) has been tempered by procedural morbidity and LVOT obstruction risk in some patients.
For clinicians, the study suggests an additional therapeutic pathway for patients with severe MR deemed unsuitable for repair or surgery. Patient selection remains critical: anatomic suitability for device anchoring, assessment of LVOT geometry, annular dimensions, and the multidisciplinary Heart Team review were core components of eligibility. The transseptal approach employed in ENCIRCLE avoids thoracotomy and transapical access, which may reduce procedural invasiveness and improve recovery in elderly, frail patients.
Expert Commentary — Strengths, Limitations, and Open Questions
Strengths of the study include its prospective, multicentre design across multiple healthcare systems, a sizable number of treated patients for a novel device, and prespecified objective endpoints. The transseptal SAPIEN M3 design represents a logical evolution of transcatheter valve technology tailored to mitral anatomy, using a well‑known balloon‑expandable prosthesis concept adapted to the mitral position.
Limitations merit emphasis. Most importantly, ENCIRCLE is a single‑arm trial without a randomized control group. Although the observed composite outcome was substantially lower than the prespecified performance goal, historical or performance goal comparisons are susceptible to selection bias and differences in patient management. Follow‑up beyond 1 year is limited at present, and durability of TMVR prostheses in the mitral position (exposure to high‑pressure, high‑motion environment) remains an open question. Potential device‑specific issues that require longer observation include structural valve deterioration, thrombosis, paravalvular leakage, hemolysis, and the need for chronic anticoagulation. The report did not (in the summary provided here) detail rates of stroke, vascular complications, conduction disturbances, or quality‑of‑life outcomes; these are critical for comprehensive assessment.
Industry sponsorship by Edwards Lifesciences is appropriate for device development but necessitates independent confirmatory studies and transparency in data reporting. Future work should pursue randomized comparisons against standard care pathways (optimal medical therapy, TEER when feasible), head‑to‑head comparisons with other TMVR platforms where anatomically appropriate, and long‑term (>5 year) registry and core‑lab adjudicated outcomes to characterise durability and late complications.
How This Fits with Current Evidence and Guidelines
Current American and European guidelines prioritise surgical repair/replacement for severe primary MR when feasible and recommend TEER for selected patients with appropriate anatomy and elevated surgical risk (AHA/ACC 2020; ESC/EACTS 2021/2022). Randomized TEER studies in functional MR (COAPT and MITRA‑FR) demonstrated that outcomes differ by patient selection and that TEER can reduce heart‑failure admissions and mortality in carefully selected cohorts. Despite these advances, a meaningful subset of patients remains without durable transcatheter or surgical options. TMVR technologies, especially via less invasive transseptal access, are intended to fill this gap, and ENCIRCLE provides important early evidence supporting SAPIEN M3 as an option in this population. However, guideline adoption of TMVR will require randomized and long‑term data to define comparative effectiveness and durability relative to existing therapies.
Practical Takeaways for Clinicians
– For patients with symptomatic severe MR who are contraindicated for surgery and unsuitable for TEER, transseptal TMVR with SAPIEN M3 appears to be a viable option that may reduce death or heart‑failure rehospitalisation at 1 year in appropriately selected patients.
– Multidisciplinary Heart Team assessment, careful preprocedural imaging (including CT to assess LVOT risk and annular geometry), and attention to anticoagulation planning are essential to optimise outcomes and mitigate device‑specific risks.
– Shared decision‑making with patients should include discussion of the novel and evolving nature of TMVR, uncertainties about long‑term durability, and alternatives including palliative medical therapy or enrolment in clinical trials offering randomised comparisons where available.
Conclusion
The ENCIRCLE pivotal trial demonstrates that percutaneous transseptal TMVR using the SAPIEN M3 system can be delivered to a high‑risk population with symptomatic moderate‑to‑severe or severe MR who are not candidates for surgery or TEER, producing a substantially lower rate of the composite of death or heart‑failure rehospitalisation at 1 year than the prespecified performance goal. These data support expanding the therapeutic armamentarium for patients lacking other options, but confirmatory randomized trials, longer follow‑up, and real‑world registry evidence will be needed to define the role of transseptal TMVR in practice and to characterise durability and late safety outcomes.
Funding and ClinicalTrials.gov
ENCIRCLE was funded by Edwards Lifesciences. ClinicalTrials.gov registration: NCT04153292.
References
1. Guerrero ME, Daniels DV, Makkar RR, et al.; ENCIRCLE Trial Executive Committee and Study Investigators. Percutaneous transcatheter valve replacement in individuals with mitral regurgitation unsuitable for surgery or transcatheter edge‑to‑edge repair: a prospective, multicountry, single‑arm trial. Lancet. 2025 Oct 27:S0140‑6736(25)02073‑2. doi: 10.1016/S0140‑6736(25)02073‑2. Epub ahead of print.
2. Otto CM, Nishimura RA, Bonow RO, et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: Executive Summary. Circulation. 2021;143(5):e35–e71. doi:10.1161/CIR.0000000000000923.
3. Vahanian A, Beyersdorf F, Praz F, et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2022;43(7):561–632. doi:10.1093/eurheartj/ehab395.
4. Feldman T, Kar S, Rinaldi M, et al. Percutaneous Mitral Repair with the MitraClip System. N Engl J Med. 2011;364(15):1395–1406. doi:10.1056/NEJMoa1008823.
5. Stone GW, Lindenfeld J, Abraham WT, et al. Transcatheter Mitral‑Valve Repair in Patients with Heart Failure. N Engl J Med. 2018;379(24):2307–2318. (COAPT trial). doi:10.1056/NEJMoa1806640.
6. Obadia JF, Messika‑Zeitoun D, Leurent G, et al. Percutaneous Repair or Medical Treatment for Secondary Mitral Regurgitation. N Engl J Med. 2018;379(24):2297–2306. (MITRA‑FR trial). doi:10.1056/NEJMoa1805374.
Thumbnail image prompt
A detailed, realistic medical illustration of a transseptal mitral valve replacement procedure in a modern catheterisation lab: fluoroscopy and transesophageal echo overlays showing a catheter crossing the interatrial septum and deploying a prosthetic mitral valve; calm neutral colors, clear anatomical labels, clinician team in scrubs in the background, conveying technical precision and patient safety.