Highlights
– A large multi‑center study (Zhang et al., Advanced Science, 25 July 2025) found PTFE microplastics in the male urogenital system at a high detection rate (46.62%) and evidence of biological accumulation.
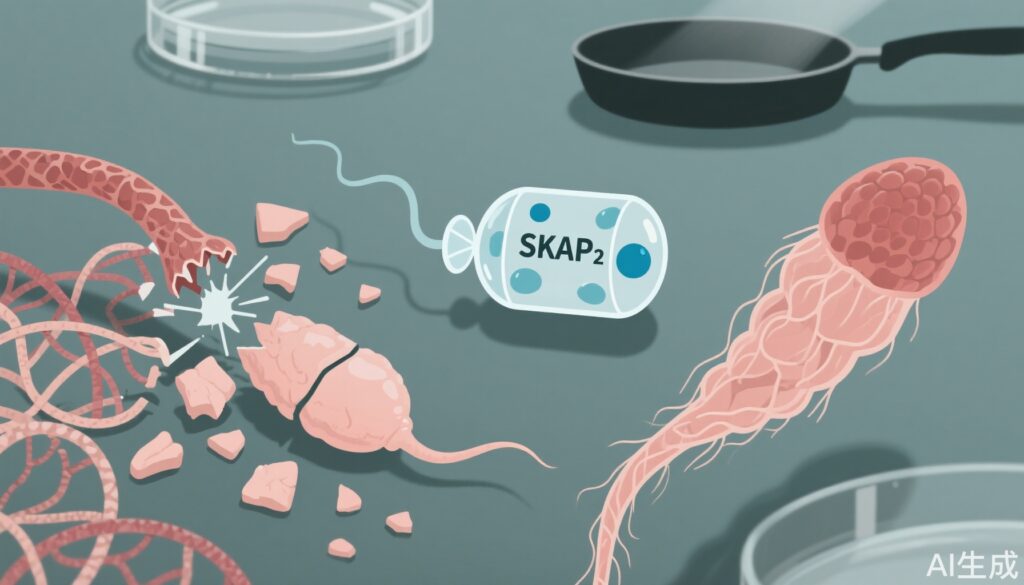
– PTFE exposure delayed spermatogonial and primary spermatocyte development, disrupted meiotic chromosome pairing, impaired DNA damage responses, and promoted spermatocyte apoptosis.
– PTFE selectively downregulated SKAP2 in haploid spermatids, causing cytoskeletal disruption, abnormal sperm morphology and reduced motility; SKAP2‑enriched milk‑derived extracellular vesicles (mEVs‑SKAP2) restored cytoskeletal integrity, sperm motility and DNA integrity in human samples and improved fertility outcomes in mice.
Study background and disease burden
Male infertility attributable to impaired spermatogenesis is a growing global public‑health concern. Clinically, declining sperm concentration, increased rates of teratozoospermia (abnormal morphology), and asthenozoospermia (reduced motility) have been reported in many regions and are linked to couple infertility, increased need for assisted reproductive technology, and psychological and economic burden.
Environmental exposures are increasingly recognized contributors to poor sperm quality. Microplastics are ubiquitous in air, water, food and consumer products; several studies have documented microplastic presence in human tissues (for example, microplastics in human placenta) and in circulation, raising concerns about reproductive toxicity. Polytetrafluoroethylene (PTFE, commercial name: Teflon) is a widely used fluoropolymer with thermal stability that makes it common in cookware and certain medical devices. Prior to the study by Zhang et al., mechanistic data directly linking PTFE microplastic exposure to human spermatogenic damage were sparse.
Study design
Zhang et al. performed an integrated investigation combining human epidemiologic sampling, ex vivo human sperm assays, single‑cell and bulk transcriptomics, and mouse in vivo exposure models. Key components included:
– Human sampling and detection: measurement of PTFE burden in male urogenital tissues/fluids and correlation with semen parameters; the stated detection rate of PTFE in the male urogenital system was 46.62%.
– Cellular and molecular analysis: single‑cell transcriptomics across spermatogenic lineages to identify cell types and transcriptional programs affected by PTFE; assessment of meiotic chromosome pairing, DNA damage response markers, and apoptosis in germ cells.
– Mechanistic interrogation: evaluation of SKAP2 expression across germ‑cell stages and functional assays linking SKAP2 loss to actin cytoskeleton disruption in haploid spermatids.
– Therapeutic exploration: isolation of milk‑derived extracellular vesicles (mEVs), engineering or enrichment of these vesicles with SKAP2 (mEVs‑SKAP2), and testing their ability to restore cytoskeletal structure, sperm morphology, motility and DNA integrity in human sperm samples and in PTFE‑exposed mouse models. Fertility endpoints (e.g., mating success, litter size) were reported in mice.
Primary endpoints included changes in sperm morphology, motility and DNA integrity; secondary endpoints included markers of meiotic progression and apoptosis in germ cells, as well as fertility outcomes in animal models.
Key findings
PTFE detection and bioaccumulation
– PTFE was detected in the male urogenital system in a sizable fraction of sampled individuals (46.62%), indicating exposure and potential local accumulation. The authors emphasize that this is likely underrecognized in routine environmental assessments because PTFE is relatively inert and historically understudied as a microplastic of reproductive concern.
Effects on spermatogenesis
– Developmental delay: PTFE exposure delayed progression of spermatogonia and primary spermatocytes, with reduced representation of later spermatogenic stages.
– Meiotic defects: PTFE‑exposed germ cells demonstrated defective chromosome synapsis (impaired homolog pairing) during meiosis, a critical step for accurate segregation and genetic integrity.
– DNA damage response (DDR) impairment: canonical DDR markers were dysregulated in PTFE‑exposed testes, indicating reduced capacity to recognize or repair double‑strand breaks occurring during meiosis; this was associated with increased apoptosis among primary spermatocytes.
SKAP2 as a specific target and mechanism
– Cell‑type specificity: single‑cell transcriptome profiling revealed selective downregulation of SKAP2 (Src kinase‑associated phosphoprotein 2) in haploid spermatids after PTFE exposure.
– Functional consequences: SKAP2 is known to interact with nucleation‑promoting factors (NPFs) such as WAVE family proteins to regulate F‑actin assembly. The authors demonstrated that SKAP2 deficiency in spermatids led to disorganized F‑actin structures, abnormal head‑tail junction architecture, misshapen sperm heads and impaired flagellar ultrastructure, producing typical morphological abnormalities and reduced progressive motility.
Therapeutic rescue with mEVs‑SKAP2
– In ex vivo human sperm assays and in vivo mouse models, administration of milk‑derived extracellular vesicles enriched for SKAP2 (mEVs‑SKAP2) repaired cytoskeletal architecture in haploid germ cells and mature spermatozoa.
– Functional restoration: treated human spermatozoa and PTFE‑exposed mice exhibited improved sperm motility and progressive movement, improved sperm morphology scores, and improved measures of DNA integrity compared with untreated PTFE‑exposed controls. In mice, fertility (mating success and litter size) was partially or fully restored depending on dosing and timing.
– Specificity: the therapeutic effect correlated with SKAP2 content of EVs; control EVs without SKAP2 enrichment had minimal restorative activity, supporting SKAP2 as a key effector.
Safety and tolerability (preclinical)
– The paper reports no acute local toxicity to testes from intratesticular or systemic administration of mEVs at the tested doses in mice. Long‑term safety and immunogenicity assessments required for translational development were identified as outstanding needs.
Statistical reporting
– The authors report statistically significant differences between PTFE‑exposed and control groups for key endpoints (p < 0.05). Specific effect sizes and confidence intervals are reported in the original paper for each assay; the reported 46.62% PTFE detection rate is a key epidemiologic figure.
Expert commentary
Biological plausibility and novelty
– The proposed mechanism—environmental microplastic exposure leading to selective downregulation of a cytoskeletal regulator (SKAP2) in haploid spermatids with consequent structural and functional sperm defects—is biologically plausible. F‑actin dynamics are crucial for spermiogenesis, shaping of the sperm head and formation of the head‑tail coupling apparatus.
Strengths
– The study integrates human sampling, high‑resolution single‑cell transcriptomics, mechanistic cellular assays and in vivo models, strengthening causal inference beyond simple association.
– The translational therapeutic approach—use of extracellular vesicles as a delivery system for a protein regulator—is innovative and leverages natural intercellular communication mechanisms.
Limitations and uncertainties
– Exposure assessment: while a 46.62% detection rate is striking, the sampling strategy, representativeness and dose‑response relationships require broader validation across populations and geographies.
– Causality in humans: the human component includes correlation and ex vivo testing; definitive causal demonstration in humans will require longitudinal exposure data and interventional trials.
– Therapeutic translation: mEVs‑SKAP2 were effective in preclinical models, but clinical translation faces multiple hurdles: scalable and standardized EV production, dose‑finding, biodistribution, long‑term safety, and regulatory classification (biologic vs. cell therapy product). Immunogenicity of exogenous protein cargo and off‑target effects must be addressed.
– Specificity: while SKAP2 appears central in these models, other pathways may also contribute to PTFE toxicity; combinatorial or upstream interventions might be required.
Context with existing literature
– This study builds on growing evidence that microplastics can reach human tissues and potentially disrupt physiology (for example, detection of microplastics in human placenta and blood reported in recent years). It expands the field by pinpointing a molecular target and offering a potential restorative strategy.
Conclusion
Zhang et al. provide compelling preclinical and translational evidence that PTFE microplastic exposure is associated with disrupted spermatogenesis via downregulation of SKAP2 in haploid germ cells, leading to cytoskeletal damage, abnormal sperm morphology and loss of motility. Importantly, they demonstrate that SKAP2‑enriched milk‑derived extracellular vesicles can restore cytoskeletal integrity and sperm function in human samples and mouse models, and improve fertility outcomes in mice.
Clinical and public‑health implications
– For clinicians, these findings underscore the potential contribution of environmental PTFE exposure to otherwise unexplained declines in sperm quality and point to SKAP2 and actin cytoskeleton pathways as targets for future diagnostic and therapeutic development.
– From a public‑health perspective, the detection of PTFE in the male urogenital system argues for renewed attention to consumer exposures (e.g., high‑temperature cooking with non‑stick cookware), occupational safeguards, and environmental policy to reduce microplastic burden.
Next steps and research needs
– Replication of epidemiologic findings in independent cohorts with quantified exposure assessment.
– Dose‑response and temporality studies to better define windows of vulnerability.
– Preclinical GLP toxicology, pharmacokinetics and immunogenicity studies of mEVs‑SKAP2.
– Early‑phase human trials focused on safety, dosing and biomarker endpoints (sperm motility and DNA integrity) before proceeding to fertility outcomes.
In summary, the study adds a mechanistic link between PTFE microplastics and impaired spermatogenesis and introduces a novel, biologically rational EV‑based repair strategy. The work is an important step toward translation but requires careful validation and safety evaluation before clinical use.
References
– Zhang C, Huang H, Li Y, Ren J, Gan S, et al. Therapeutic Repair of Sperm Quality Decline Caused by Polytetrafluoroethylene. Advanced Science. 2025 Jul 25. (Article as described in the prompt.)
– Wright SL, Kelly FJ. Plastic and Human Health: A Micro Issue? Environmental Science & Technology. 2017;51(12):6634–6647.
– Ragusa A, Svelato A, Santacroce C, et al. Plasticenta: First evidence of microplastics in human placenta. Environment International. 2021;146:106274.
(Readers are encouraged to consult the original Advanced Science paper for full methods, numerical effect sizes, statistical analyses and supplementary data.)