Posted inClinical Updates news Public Health Respiratory
Durability and Decay: Evaluating Long-term RSV Vaccine Effectiveness in a Large Real-World Cohort of US Veterans
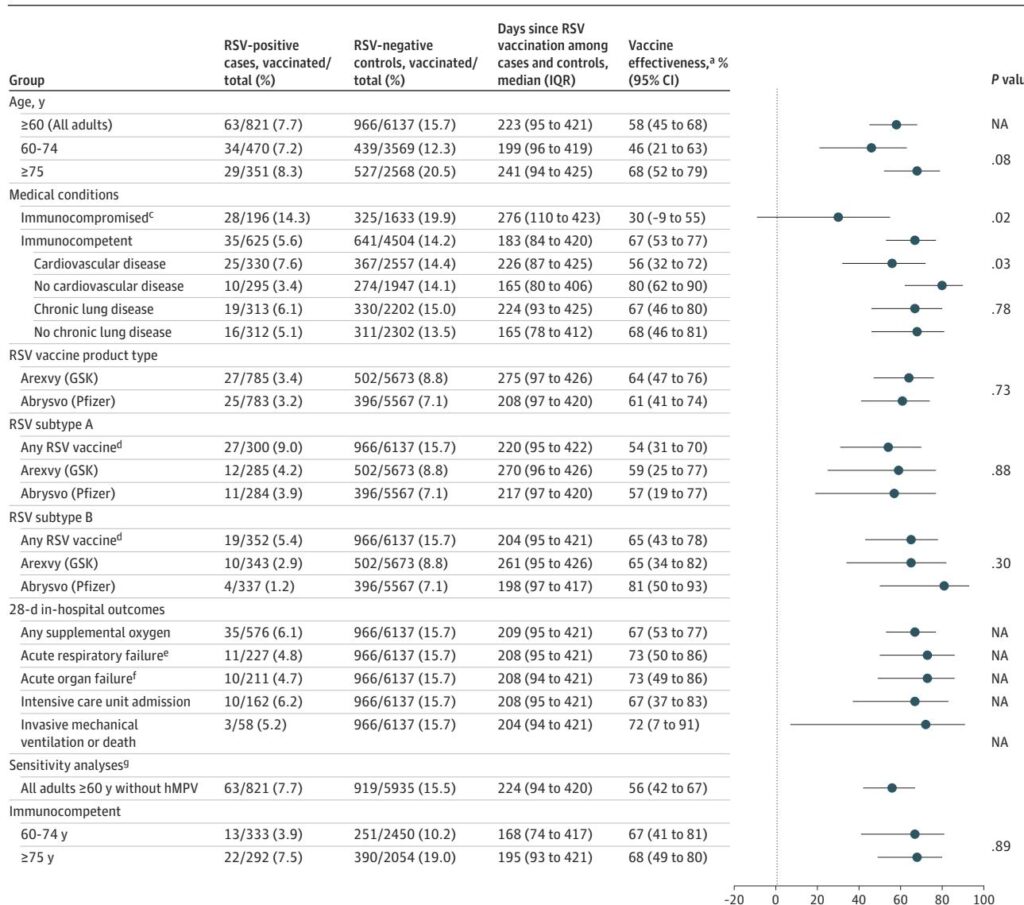
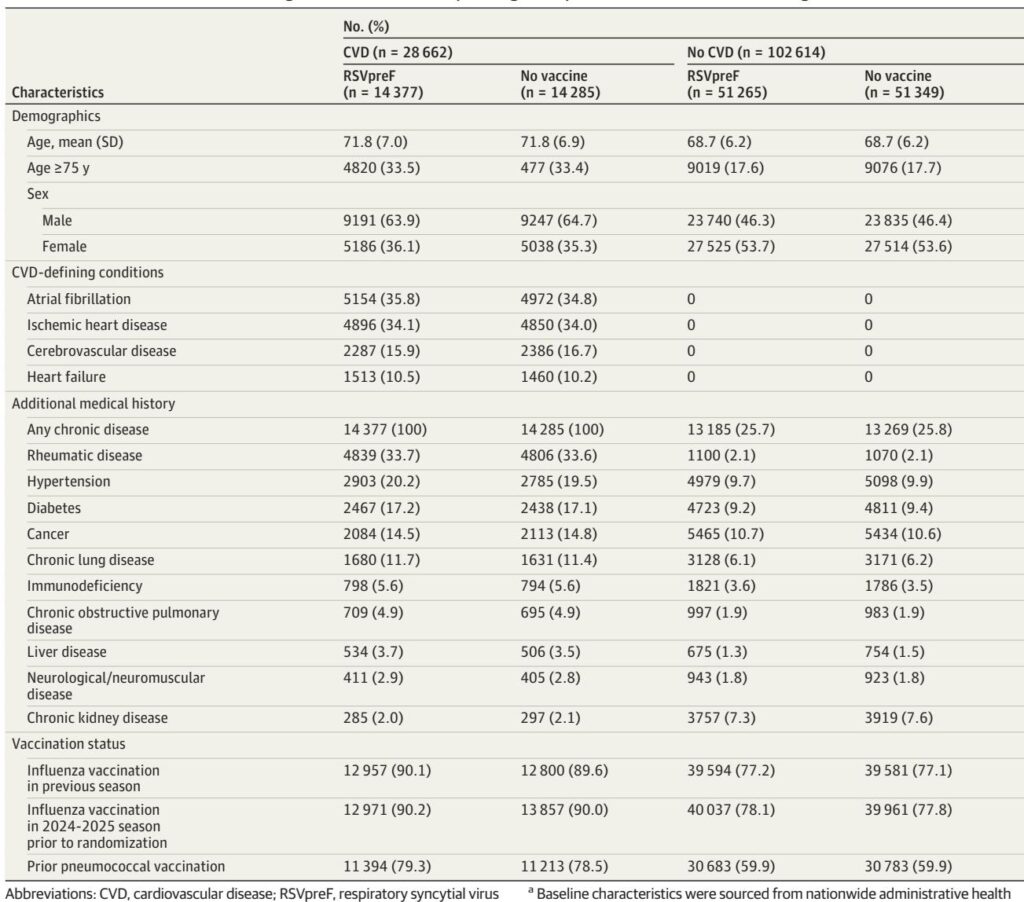
This review analyzes the long-term effectiveness of RSV vaccination among US Veterans, revealing significant protection against severe outcomes that wanes over two seasons, particularly in immunocompromised populations.