Background and Disease Burden
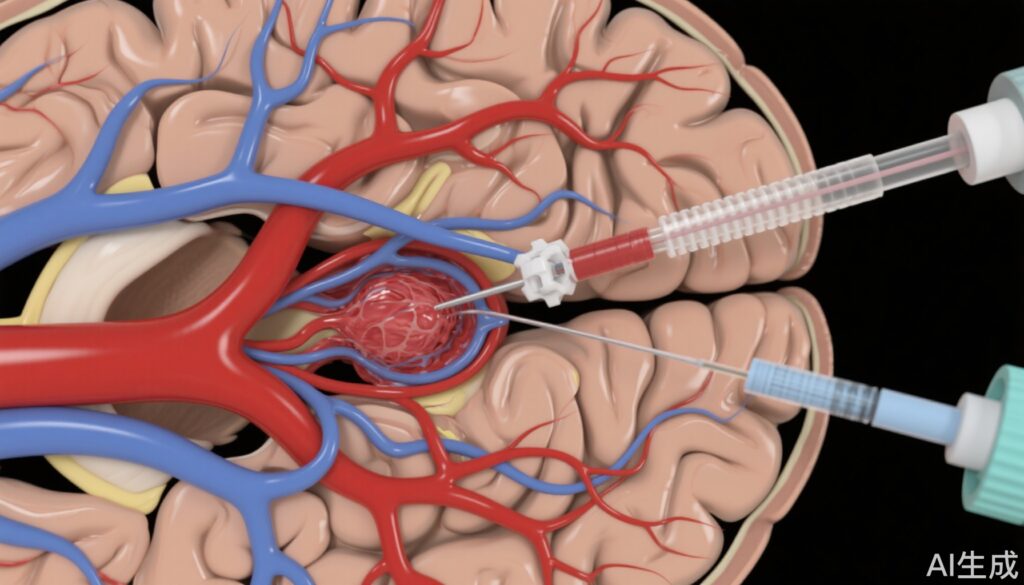
Acute ischemic stroke caused by large vessel occlusion (LVO), particularly in the anterior circulation involving the internal carotid artery (ICA) and middle cerebral artery (MCA) M1 segment, remains a leading cause of mortality and long-term disability worldwide. Rapid and complete recanalization is crucial to preserve cerebral tissue and improve functional outcomes. Mechanical thrombectomy using aspiration catheters and stent retrievers has revolutionized the management of LVO strokes. However, limitations in device design, such as the inner diameter of aspiration catheters, may influence successful reperfusion rates and procedural safety.
The development of super large bore aspiration catheters (0.088″ inner diameter) promises to enhance thrombus ingestion capacity and reperfusion efficacy. Despite promising clinical experiences, no randomized controlled trial has yet evaluated the safety and effectiveness of these devices compared to established systems. This evidence gap prompted the SUMMIT MAX trial to assess the Route 92 Medical HiPoint Reperfusion System versus the Vecta Aspiration System (Stryker Neurovascular) in patients with anterior circulation LVO.
Study Design
SUMMIT MAX was a prospective, randomized, controlled, open-label trial enrolling 250 patients presenting with acute ischemic stroke due to internal carotid artery or middle cerebral artery M1 occlusions. Eligible patients underwent aspiration thrombectomy with either the super large bore HiPoint Reperfusion System or the widely used Vecta Aspiration System. A modified intent-to-treat population of 166 patients (89 in HiPoint group, 77 in Vecta group) was analyzed.
The primary effectiveness endpoint was achieving successful reperfusion defined as modified Treatment in Cerebral Infarction (mTICI) score ≥2b, adjudicated by an independent core laboratory. Importantly, reperfusion success required use of only the assigned device; any adjunctive devices before or after use of the study device defined failure. The primary safety endpoint was symptomatic intracranial hemorrhage within 24 hours post-procedure. Secondary endpoints included 90-day clinical outcomes measured by modified Rankin Scale (mRS) scores.
The trial tested noninferiority of the HiPoint system compared to the Vecta system with a noninferiority margin of 12.5%.
Key Findings
The median age in the studied population was 69 years with 54.2% female patients. The HiPoint system demonstrated a significantly higher rate of successful reperfusion using only the assigned device without adjunctive therapies: 77.5% (69/89) versus 50.6% (39/77) for Vecta (P<0.0001 for noninferiority). This result indicates clear noninferiority and suggests potential superiority in effective clot retrieval attributable to the larger 0.088" inner diameter of the HiPoint system.
Safety profiles were comparable between the two groups. Symptomatic intracranial hemorrhage incidence within 24 hours was low and statistically similar (3.6% in HiPoint vs 2.7% in Vecta). This finding confirms that the super large bore design does not increase hemorrhagic complications.
At 90 days, favorable clinical outcomes (mRS ≤2) were observed in 50.6% of patients treated with HiPoint and 53.3% with Vecta, with no statistically significant difference (absolute difference -2.8%, 95% CI -18.2 to 12.7%; P=0.75). Thus, despite improved reperfusion rates, clinical outcomes were equivalent, possibly reflecting multifactorial influences beyond reperfusion alone.
Expert Commentary
The SUMMIT MAX trial provides robust evidence supporting the safety and efficacy of the super large bore HiPoint Reperfusion System for aspiration thrombectomy in anterior circulation LVO strokes. The higher rates of successful reperfusion with the HiPoint system suggest that larger catheter inner diameters may facilitate more effective clot engagement and removal.
However, the lack of significant difference in 90-day functional outcomes underscores that clinical recovery after stroke depends on factors beyond recanalization, including patient comorbidities, collateral circulation, and time-to-treatment. Experts emphasize the importance of integrating device innovation with optimal workflow and comprehensive patient management.
Limitations include an open-label design and strict criteria defining success, which might have underestimated real-world efficacy when adjunctive devices are used. Future studies may explore combination strategies or assess device performance across diverse vascular anatomies.
Conclusion
The SUMMIT MAX randomized trial establishes that the super large bore HiPoint Reperfusion System is noninferior to the Vecta Aspiration System for aspiration thrombectomy in large vessel occlusion stroke, with a superior successful reperfusion rate and comparable safety. These findings support wider adoption of large bore catheters for mechanical thrombectomy, potentially enhancing acute stroke care efficacy.
Continued research should focus on optimizing patient selection and integrating device innovation with comprehensive stroke networks to improve functional recovery outcomes.
References
Nguyen TN, Dabus G, McGuinness B, et al. SUMMIT MAX: A Randomized Trial of the Super Large Bore HiPoint Reperfusion System Versus Vecta System for Aspiration Thrombectomy. Stroke. 2025 Aug;56(8):1980-1990. doi: 10.1161/STROKEAHA.125.051742. Epub 2025 May 21. PMID: 40395106.