Highlights
No Reduction in Ventilator Complications
The use of polyurethane-cuffed endotracheal tubes with subglottic suction (PU-EVAC) did not result in a statistically significant reduction in infection-related ventilator-associated complications (IVAC) or possible ventilator-associated pneumonia (VAP) compared to standard polyvinylchloride (PVC) tubes.
Long-Term Outcomes Comparable
At the 6-month follow-up, there were no significant differences between the PU-EVAC and PVC groups regarding laryngeal injury, cognitive function, or physical and mental quality-of-life scores, although high attrition rates in survivors necessitate cautious interpretation.
Safety and Mortality
Mortality rates at six months were high in both cohorts (exceeding 50%), reflecting the severity of illness in patients requiring emergency intubation. The incidence of laryngeal injury was numerically higher in the PU-EVAC group, though not reaching statistical significance.
Background: The Challenge of Microaspiration in the ICU
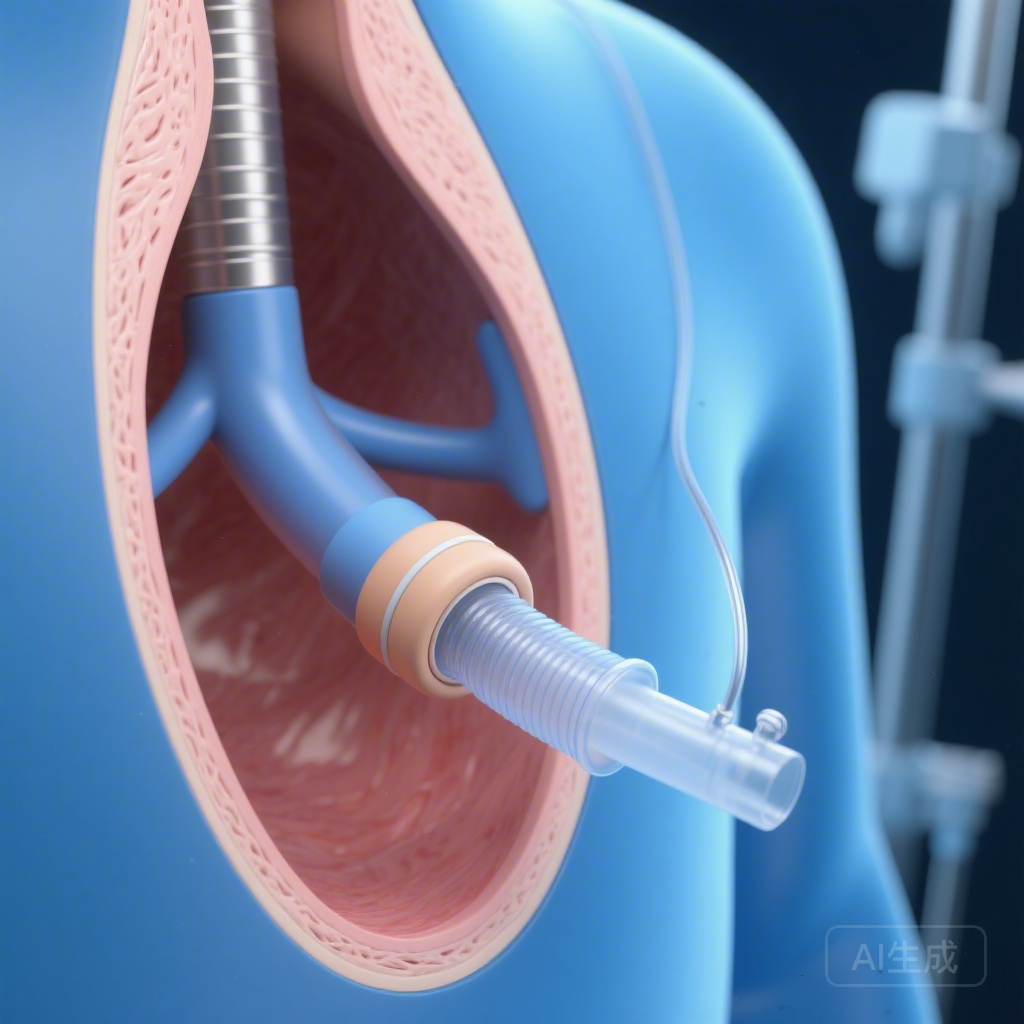
Ventilator-associated pneumonia (VAP) remains a significant cause of morbidity, mortality, and increased healthcare costs in the intensive care unit (ICU). A primary pathophysiological mechanism for VAP is the microaspiration of oropharyngeal secretions that accumulate above the endotracheal tube (ETT) cuff. Historically, two primary technological interventions have been proposed to mitigate this risk: the use of polyurethane cuffs, which are thinner and theoretically provide a better seal against the tracheal wall than traditional polyvinylchloride (PVC) cuffs, and subglottic suctioning ports, which allow for the continuous or intermittent drainage of pooled secretions.
While various clinical guidelines recommend these features for patients expected to require prolonged mechanical ventilation, their efficacy and safety in the context of emergency intubation—where the risk of airway trauma is higher and the duration of ventilation is unpredictable—have remained uncertain. The PreVent 2 trial was designed to address this evidence gap by evaluating both short-term hospital outcomes and long-term functional recovery.
Study Design and Methodology
PreVent 2 was a randomized controlled phase 2 trial conducted at two major academic centers in the United States: Oregon Health and Science University (OHSU) and Yale New Haven Hospital (YNHH). The study enrolled 1074 adult patients requiring emergency endotracheal intubation in either the emergency department (ED) or inpatient wards for acute respiratory failure.
Interventions and Comparators
Patients were randomized 1:1 to one of two groups:
- PU-EVAC Group: Received a polyurethane-cuffed endotracheal tube with a subglottic suction port. These patients received continuous subglottic suctioning until extubation.
- PVC Group: Received a standard polyvinylchloride-cuffed endotracheal tube with no subglottic drainage, following the usual standard of care.
Endpoints
The study utilized a comprehensive set of coprimary endpoints evaluated at 6 months post-intubation: laryngeal injury (assessed via endoscopic or clinical evaluation), quality of life (using the SF-36 physical and mental component summaries), and cognitive function. Secondary endpoints focused on acute hospital complications, specifically infection-related ventilator-associated complications (IVAC) and possible VAP, as defined by the Centers for Disease Control and Prevention (CDC) criteria.
Key Findings: Hospital and Long-Term Results
The trial randomized 1068 patients into the final analysis (535 in the PU-EVAC group and 533 in the PVC group). The cohort was characterized by a mean age of 62.9 years and a high baseline severity of illness.
Ventilator-Associated Complications
The primary hypothesis that PU-EVAC tubes would reduce respiratory infections was not supported by the data. IVAC occurred in 8% of the PU-EVAC group compared to 6% in the PVC group (risk difference 0.02; 95% CI -0.01 to 0.05). Similarly, possible VAP was recorded in 6% of the PU-EVAC group versus 5% in the PVC group. These findings suggest that in the acute, emergency setting, the theoretical benefits of polyurethane cuffs and subglottic suction do not translate into a measurable reduction in pulmonary infections.
Laryngeal Injury and Safety
One of the most concerning findings was the high rate of laryngeal injury observed in both groups at the 6-month follow-up. Among survivors who underwent evaluation, 83% of the PU-EVAC group and 70% of the PVC group exhibited some form of laryngeal injury. While the difference was not statistically significant (p=0.098), the numerically higher incidence in the PU-EVAC group raises questions about whether the specialized tubes, which are often bulkier or stiffer due to the suction lumen, might increase the risk of mucosal trauma during emergency placement.
Quality of Life and Cognitive Function
At 6 months, mortality was high, with 51% of the PU-EVAC group and 53% of the PVC group having died. Among the survivors who completed the 6-month follow-up (n=157), there were no significant differences in quality-of-life scores. The mean Physical Component Summary (PCS) was 39.97 for PU-EVAC and 40.49 for PVC. Cognitive impairment was prevalent in both groups (85% vs 81%), highlighting the profound long-term burden of critical illness regardless of the type of endotracheal tube used.
Expert Commentary and Clinical Interpretation
The results of PreVent 2 challenge the routine adoption of subglottic suctioning tubes in the emergency department. Several factors may explain why the expected benefits did not materialize. First, in emergency intubations, the initial insult—potential aspiration during the event itself or trauma during a difficult airway maneuver—may outweigh the subsequent benefits of subglottic drainage. Unlike elective intubations in the operating room, emergency cases often involve patients with full stomachs or high-risk physiology.
Furthermore, the physical properties of the polyurethane cuff, while superior in bench studies for preventing fluid leak, may not be sufficient to overcome the dynamic challenges of a critically ill patient who is frequently repositioned or who has significant tracheal secretions. The high rate of laryngeal injury across both groups is a stark reminder of the long-term morbidity associated with invasive mechanical ventilation. Clinicians must weigh the theoretical reduction in VAP against the potential for increased airway trauma, especially given that this trial showed no benefit in infection rates.
The study’s significant limitation—the low number of survivors available for 6-month functional testing—is a common hurdle in critical care research but one that limits the definitive nature of the long-term outcome data. However, the lack of even a signal toward benefit in the secondary infection endpoints suggests that for many centers, the higher cost of PU-EVAC tubes may not be justified for routine emergency use.
Conclusion
The PreVent 2 trial provides high-quality evidence that the use of polyurethane-cuffed endotracheal tubes with subglottic suction does not improve hospital or 6-month outcomes for patients undergoing emergency intubation. Given the lack of reduction in ventilator-associated complications and the potential for increased laryngeal injury, standard PVC tubes remain a reasonable and cost-effective standard of care in the emergency setting. Future research should focus on identifying specific patient subgroups that might still benefit from advanced ETT designs and improving the long-term survivorship and functional recovery of the critically ill.
Funding and clinicaltrials.gov
This study was funded by the National Institutes of Health (NIH) and the National Heart, Lung, and Blood Institute (NHLBI). The trial is registered at ClinicalTrials.gov under the identifier NCT03705286.
References
Treggiari MM, Sharp ES, Ohnuma T, et al. Hospital and long-term outcomes for subglottic suction and polyurethane cuff versus standard endotracheal tubes in emergency intubation (PreVent 2): a randomised controlled phase 2 trial. Lancet Respir Med. 2026 Feb;14(2):141-150. doi: 10.1016/S2213-2600(25)00294-2. Epub 2025 Nov 27. PMID: 41319662.