Highlights
- Subcutaneous ICDs (S-ICDs) significantly reduce lead-related complications compared with transvenous ICDs (TV-ICDs), especially over long-term follow-up.
- Inappropriate shocks occur more frequently with S-ICDs primarily due to cardiac oversensing and electromagnetic interference, whereas TV-ICDs have higher inappropriate shocks related to atrial arrhythmias.
- Appropriate shock efficacy of S-ICDs is comparable to TV-ICDs, despite the lack of antitachycardia pacing in S-ICDs, although S-ICD recipients may receive more shocks.
- Device selection should be individualized, considering pacing needs, risk of lead-related complications, and the causes of inappropriate shocks.
Background
Implantable cardioverter-defibrillators (ICDs) represent a cornerstone in the prevention of sudden cardiac death among patients with ventricular arrhythmias or at risk thereof. Conventional transvenous ICDs (TV-ICDs) employ intravascular leads, which provide both defibrillation and pacing capabilities but carry risks of lead-related complications including fracture, infection, and vascular damage. To mitigate these risks, subcutaneous ICDs (S-ICDs), implanted entirely extrathoracically without transvenous leads, were developed.
While S-ICDs successfully reduce lead-related complications, their comparative performance with TV-ICDs regarding inappropriate shocks and overall device-related complications has been under investigation. The landmark PRAETORIAN and ATLAS trials, along with comprehensive meta-analyses, provide pivotal data to inform clinical decision-making regarding device selection.
Key Content
Comparative Safety and Device-Related Complications
The PRAETORIAN trial and subsequent PRAETORIAN-XL extended follow-up investigated long-term safety outcomes of S-ICD versus TV-ICD therapy. The initial PRAETORIAN trial (median follow-up 49.1 months) demonstrated noninferiority of the S-ICD to the TV-ICD for a composite outcome of device-related complications and inappropriate shocks [Olde Nordkamp et al., N Engl J Med 2020].
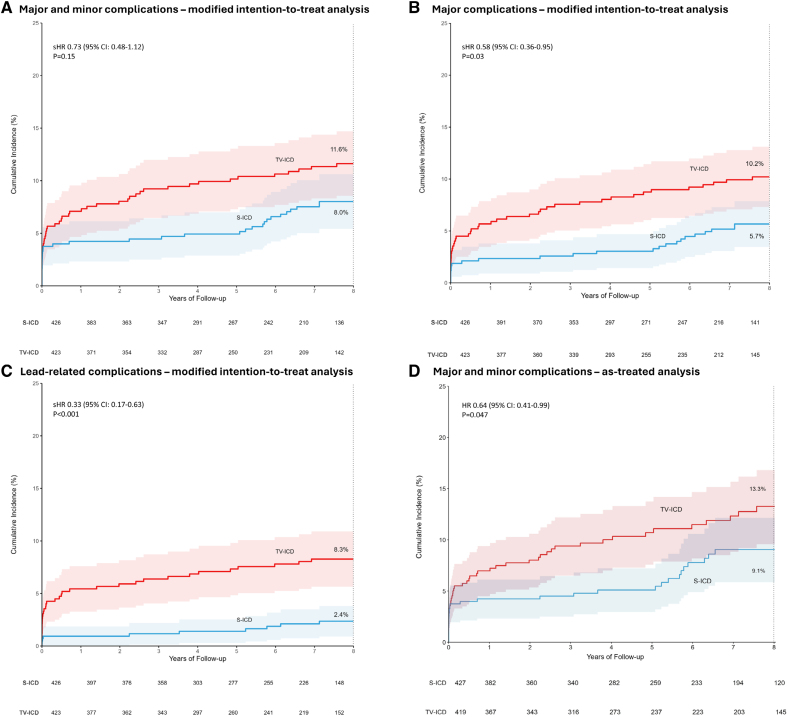
The PRAETORIAN-XL trial extended this follow-up to a median of 87.5 months and found no significant difference in the overall device-related complications between the devices; however, patients with TV-ICDs had significantly more major complications and lead-related complications requiring invasive interventions (P=0.03 and P<0.001, respectively) [Olde Nordkamp et al., Circulation 2025]. Importantly, lead-related complications—such as lead fracture or dislodgement—were virtually absent in S-ICD recipients due to the extravascular lead design.
Other analyses support these findings, indicating that S-ICD therapy is associated with fewer lead-related complications and systemic infections but higher pocket-related issues related to the device implantation site [Benz et al., J Am Coll Cardiol 2025; Eur Heart J 2022]. These data contribute to the rationale of preferring S-ICD therapy in patients without pacing indications to minimize long-term lead-associated morbidity.
Inappropriate Shock Incidence and Mechanisms
An individual participant data meta-analysis combining PRAETORIAN and ATLAS trials with 1,342 patients found that first inappropriate shock rates were overall low but significantly higher for S-ICDs than TV-ICDs (2.5 vs. 1.5 per 100 patient-years; HR 1.61, P=0.03) [Benz et al., J Am Coll Cardiol 2025].
Mechanistic evaluation revealed that S-ICD recipients had substantially higher inappropriate shocks attributable to cardiac oversensing (HR 15.07, P<0.001) and electromagnetic interference or myopotentials (HR 8.19, P=0.005). Conversely, TV-ICD recipients more frequently experienced inappropriate shocks related to atrial arrhythmias (HR 0.37 for S-ICD, P=0.003) owing to their capacity to detect and misclassify supraventricular tachyarrhythmias.
The PRAETORIAN trial secondary analysis confirmed that inappropriate therapy rates were similar between device types (~10% at 4 years), with different predominant causes: supraventricular tachycardias were prevalent in TV-ICDs, while cardiac oversensing was more frequent in S-ICDs. Predictors of inappropriate therapy included atrial fibrillation history and high baseline heart rate for TV-ICDs and prolonged QRS duration for oversensing in S-ICDs. Importantly, interventions after the first inappropriate shock significantly curtailed recurrence in both groups [Benz et al., Circ Arrhythm Electrophysiol 2024].
Patient Characteristics in the PRAETORIAN-XL Trial

Figure 2.
Eight-year estimated cumulative incidences of device-related complications
Appropriate Shock and Antitachycardia Pacing Efficacy
The PRAETORIAN trial also evaluated the efficacy and safety of appropriate shocks and the role of antitachycardia pacing (ATP) in TV-ICDs. Over a median follow-up of 52 months, no statistically significant difference was seen in the incidence of appropriate therapy between S-ICD and TV-ICD groups (19.4% vs 17.5%, P=0.45), though more patients with S-ICD received shocks (19.2% vs 11.5%; P=0.02) [Knops et al., Circulation 2022].
The first shock efficacy was high and comparable between groups (94% for S-ICD vs 92% for TV-ICD; P=0.40). ATP in TV-ICDs successfully terminated 46% of monomorphic ventricular tachycardias but accelerated arrhythmias in 9.4%. Electrical storm occurrence tended to be higher in TV-ICD patients requiring appropriate therapy, suggesting a potential advantage for S-ICD in this aspect [Knops et al., Circulation 2022].
Quality of Life and Procedural Considerations
Quality-of-life assessments from the PRAETORIAN trial revealed no significant differences between S-ICD and TV-ICD recipients in physical functioning or mental well-being over 30 months of follow-up. However, patients experiencing recent shocks—regardless of device type—reported lower social functioning and emotional role scores, highlighting the impact of ICD shocks on patient-reported outcomes [Olde Nordkamp et al., Circ Cardiovasc Qual Outcomes 2024].
Technical aspects including implantation techniques (two-incision vs three-incision for S-ICDs) do not significantly influence complication rates or efficacy but may improve cosmetic outcomes and procedural simplicity, though randomized trials are needed to confirm long-term safety [Pacing Clin Electrophysiol 2024].
Expert Commentary
The emerging evidence from large-scale randomized trials establishes that S-ICDs offer a compelling alternative to TV-ICDs in patients without pacing indications, providing similar overall safety and efficacy but with a significantly lower risk of lead-related complications. This is clinically meaningful given the morbidity and healthcare resource utilization associated with lead revisions and infections.
The higher rate of inappropriate shocks with S-ICD—primarily due to oversensing—is an important consideration. Advances in sensing algorithms and patient selection, alongside careful post-shock management, can mitigate these risks. Conversely, TV-ICDs, while delivering ATP to reduce shock burden, expose patients to risks related to transvenous leads and a different profile of inappropriate shocks typically caused by atrial arrhythmias.
Mechanistically, the extravascular positioning of S-ICD leads predisposes to oversensing of cardiac and extracardiac signals, but avoids pacing-related complications. Therefore, in patients requiring bradycardia or antitachycardia pacing, TV-ICDs remain standard. However, most patients eligible for ICD therapy do not require pacing and may benefit from S-ICD implantation.
Guidelines increasingly endorse S-ICD use in appropriate patients, emphasizing shared decision-making that incorporates patient comorbidities, lifestyle, and preferences. Future device iterations targeting oversensing and delivering ATP capabilities could further optimize outcomes.
Conclusion
Subcutaneous implantable cardioverter-defibrillators have demonstrated noninferiority to transvenous systems in preventing sudden cardiac death, with a net clinical benefit driven by substantially reduced lead-related complications over long-term follow-up. Inappropriate shock burden, while slightly higher in S-ICDs due to oversensing phenomena, can be managed with tailored programming and follow-up interventions.
Appropriate shock efficacy is comparable between device types, though lack of ATP in S-ICDs may increase shock incidence but potentially reduces arrhythmia acceleration risks. Quality of life is not significantly different across devices, although shock experiences adversely affect patient well-being.
Collectively, the PRAETORIAN and ATLAS trials, their meta-analyses, and supporting evidence support the preferential consideration of S-ICDs in patients without pacing indications, balancing efficacy, safety, and patient-centered outcomes. Continued innovation and longer-term data are needed to refine patient selection and device technology.
References
- Benz AP, Olde Nordkamp LRA, et al. Inappropriate Shocks From Subcutaneous vs Transvenous Implantable Cardioverter-Defibrillators: Individual Participant Data Meta-Analysis of Randomized Trials. J Am Coll Cardiol. 2025 Nov 25;S0735-1097(25)09920-6. doi: 10.1016/j.jacc.2025.10.020 IF: 22.3 Q1 . PMID: 41369620 IF: 22.3 Q1 .
- Olde Nordkamp LRA, de Veld JA, et al. Device-Related Complications in Transvenous Versus Subcutaneous Defibrillator Therapy During Long-Term Follow-Up: The PRAETORIAN-XL Trial. Circulation. 2025 Jul 22;152(3):172-182. doi: 10.1161/CIRCULATIONAHA.125.074576 IF: 38.6 Q1 . PMID: 40279654 IF: 38.6 Q1 .
- Knops RE, van der Stuijt W, et al. Efficacy and Safety of Appropriate Shocks and Antitachycardia Pacing in Transvenous and Subcutaneous Implantable Defibrillators: Analysis of All Appropriate Therapy in the PRAETORIAN Trial. Circulation. 2022 Feb;145(5):321-329. doi: 10.1161/CIRCULATIONAHA.121.057816 IF: 38.6 Q1 . PMID: 34779221 IF: 38.6 Q1 .
- Olde Nordkamp LRA, et al. Inappropriate Therapy and Shock Rates Between the Subcutaneous and Transvenous Implantable Cardiac Defibrillator: A Secondary Analysis of the PRAETORIAN Trial. Circ Arrhythm Electrophysiol. 2024 Dec;17(12):e012836. doi: 10.1161/CIRCEP.124.012836 IF: 9.8 Q1 . PMID: 39624908 IF: 9.8 Q1 .
- Olde Nordkamp LRA, et al. Quality of Life in Subcutaneous or Transvenous Implantable Cardioverter-Defibrillator Patients: A Secondary Analysis of the PRAETORIAN Trial. Circ Cardiovasc Qual Outcomes. 2024 Nov;17(11):e010822. doi: 10.1161/CIRCOUTCOMES.124.010822 IF: 6.7 Q1 . PMID: 39561235 IF: 6.7 Q1 .