Introduction: The Evolution of Sudden Cardiac Death Prevention
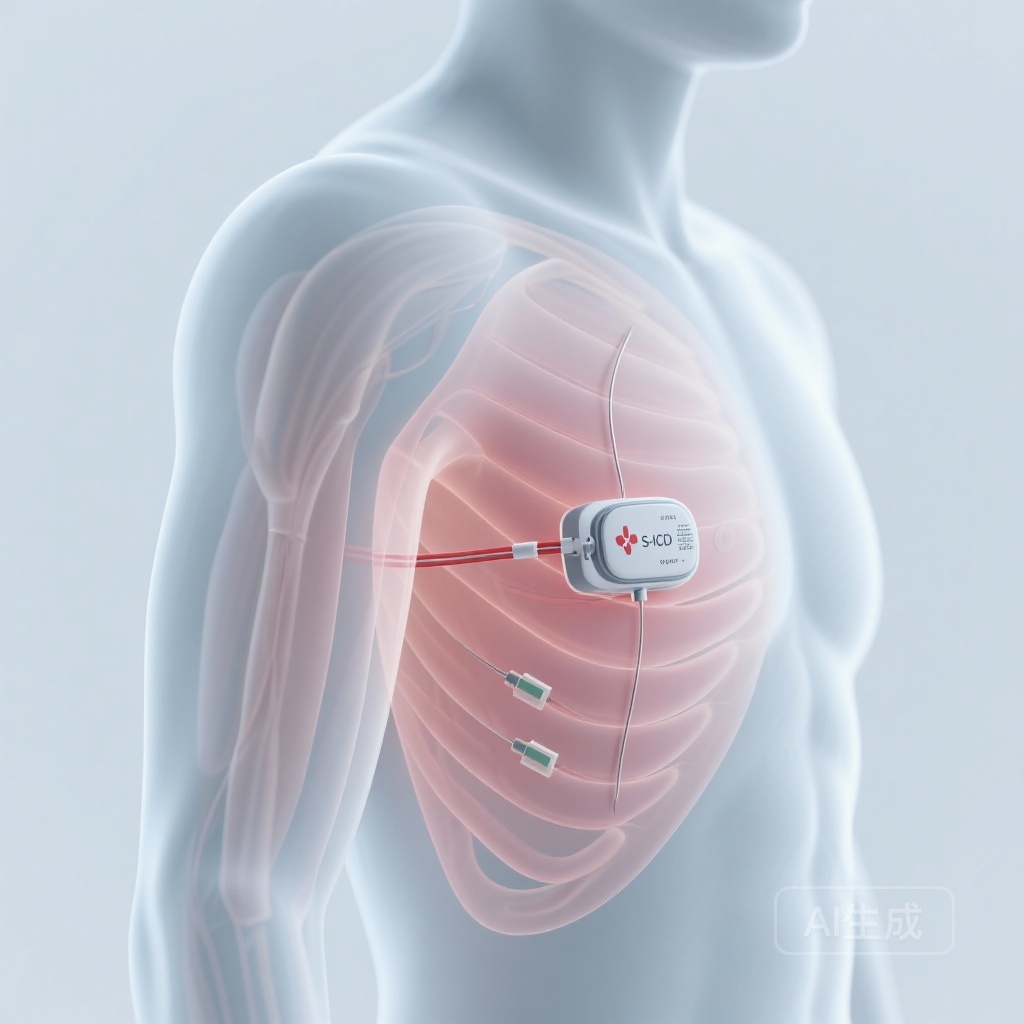
The prevention of sudden cardiac death (SCD) remains a cornerstone of modern cardiology. For decades, the transvenous implantable cardioverter-defibrillator (TV-ICD) has been the gold standard. However, the Achilles’ heel of transvenous systems—lead-related complications such as fractures, insulation failure, and systemic infections—prompted the development of the subcutaneous implantable cardioverter-defibrillator (S-ICD). By avoiding the vasculature and the heart itself, the S-ICD offers a promising alternative, particularly for younger patients or those at high risk of infection. Despite its increasing adoption, long-term data from large-scale, industry-independent populations have been relatively scarce. The HONEST (Subcutaneous Implantable Cardioverter-defibrillator: A Nationwide French Study) study, recently published in the European Heart Journal, addresses this evidence gap by providing a comprehensive look at five-year outcomes in an unselected nationwide cohort.
Study Design and Patient Demographics
The HONEST study is a prospective, academic, multicenter observational study that achieved a remarkable 98.2% enrollment rate of all patients implanted with an S-ICD across France between 2012 and 2019. This high level of inclusion minimizes selection bias and provides a true reflection of real-world clinical practice.
Population Characteristics
The study enrolled 4,924 patients with a mean age of 49.9 years. The cohort was predominantly male (76.7%), and 63.0% of the implants were for primary prevention of SCD. This demographic profile reflects the typical S-ICD candidate—often younger than the average TV-ICD recipient and frequently lacking a requirement for bradycardia pacing or cardiac resynchronization therapy.
Procedural Details
The majority of implants were performed under general anesthesia (78.9%), and defibrillation testing (DFT) was conducted in 82.6% of cases. The high rate of DFT reflects the early-to-mid-adoption phase of the technology when intraoperative verification of sensing and conversion was considered standard protocol.
Key Findings: Long-Term Performance and Safety
The five-year follow-up data from the HONEST study provides a detailed roadmap of the S-ICD’s clinical trajectory, revealing both the strengths of the technology and areas requiring further optimization.
Inappropriate Shocks: The Ongoing Challenge
The cumulative incidence of inappropriate shocks at five years was 13.8%. While this remains a significant concern, the study identified several independent risk factors and a critical mitigation strategy. Inappropriate shocks were more frequent in:
One of the most significant findings was the impact of the SMART Pass algorithm. This high-pass filter, designed to reduce T-wave oversensing, was associated with a 33% reduction in the risk of inappropriate shocks (HR 0.67, P = .007). This underscores the importance of advanced signal processing in improving the patient experience with S-ICDs.
Device Longevity and Reoperation
A notable finding in the HONEST study was the 10.8% rate of early battery depletion at five years. This rate is higher than many clinicians anticipated and highlights a potential drawback of earlier-generation S-ICD models. Consequently, the cumulative incidence of reoperation was 16.9%, driven not only by battery depletion but also by lead issues and device extractions. Definite S-ICD extraction occurred in 8.4% of patients, often due to a newly developed need for pacing (3.1%) or following complications.
Infection and Mechanical Complications
The S-ICD continued to demonstrate a favorable profile regarding systemic infection. The five-year incidence of infection was 2.4%, which is competitive with TV-ICD data, particularly considering the lack of endovascular leads. Lead dysfunction (1.5%) and chronic discomfort (1.4%) were relatively rare, suggesting that the physical presence of the device is well-tolerated by most patients over the long term.
Efficacy in Arrhythmia Termination
Critically, the S-ICD proved highly effective at its primary task: detecting and treating life-threatening ventricular arrhythmias. The incidence of ineffective shocks or undetected arrhythmias was remarkably low at only 0.2%. This confirms that despite its extracardiac location, the S-ICD is a robust tool for terminating ventricular fibrillation and flutter. However, the study did note that among patients receiving inappropriate shocks, approximately 1% experienced induced ventricular fibrillation, with one recorded fatality, highlighting the psychological and physiological toll of non-therapeutic therapy.
Expert Commentary and Clinical Implications
The HONEST study serves as a vital benchmark for cardiac electrophysiologists. The data suggest that while the S-ICD is an excellent choice for preventing SCD without the risks of transvenous leads, it is not a ‘set and forget’ technology.
Patient Selection and Counseling
The increased risk of inappropriate shocks in patients with ARVC and obesity suggests that these populations require particularly meticulous screening and programming. For patients with ARVC, the evolving nature of the substrate and the potential for changing EKG morphologies may complicate long-term sensing. Clinicians should use the SMART Pass filter routinely and consider the patient’s body habitus when determining electrode placement.
The Longevity Gap
The 10.8% early battery depletion rate is a call to action for device manufacturers. As the S-ICD population is often younger, the cumulative burden of multiple generator changes over a lifetime is significant. Improvements in battery chemistry and energy efficiency are essential to bring S-ICD longevity in line with modern TV-ICD systems.
Comparison with TV-ICDs
While HONEST was not a randomized head-to-head trial against TV-ICDs, its results align with the findings of the PRAETORIAN trial, which showed non-inferiority of the S-ICD regarding major complications and inappropriate shocks. The HONEST data reinforces the S-ICD as a primary option for patients who do not require pacing, while also reminding clinicians of the specific trade-offs involved.
Conclusion
The French nationwide HONEST study provides the most comprehensive real-world assessment of the S-ICD to date. With a 98% inclusion rate, it offers an unfiltered view of the device’s long-term performance. While the efficacy in arrhythmia termination is nearly flawless, the challenges of inappropriate shocks and battery longevity remain. The significant benefit observed with the SMART Pass algorithm provides a clear pathway for improving patient outcomes. As the technology continues to iterate—including the potential for leadless pacing integration—the S-ICD is poised to remain a vital component of the electrophysiologist’s armamentarium for years to come.
References
1. Kerkouri F, Marquié C, Boveda S, et al. Long-term outcomes of subcutaneous implantable cardioverter defibrillators: the French nationwide HONEST study. Eur Heart J. 2025;ehaf918. doi:10.1093/eurheartj/ehaf918.
2. Knops RE, Olde Nordkamp LRA, Delnoy PPHM, et al. Subcutaneous or Transvenous Defibrillator Therapy. N Engl J Med. 2020;383(6):526-536.
3. Boersma L, Barr C, Knops R, et al. Implant and Midterm Outcomes of the Subcutaneous Implantable Cardioverter-Defibrillator (S-ICD) Registry: The EFFORTLESS Study. J Am Coll Cardiol. 2017;70(7):830-841.