Highlights
– Two large retrospective cohort analyses (JAMA Dermatol. 2025; Allergy. 2025) identify consistent, often strong, positive associations between seborrheic dermatitis (SD) and a spectrum of epithelial barrier diseases (EBDs).
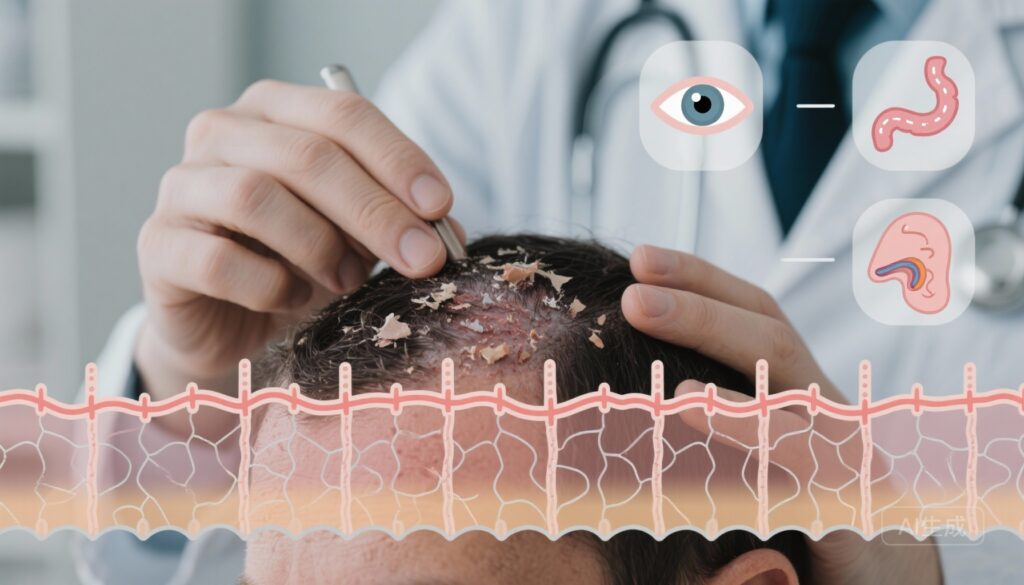
– Associations are observed across skin (atopic dermatitis, psoriasis, alopecia areata, rosacea), mucosal/ocular surfaces (dry eye, ocular allergy, rhinosinusitis), and gastrointestinal diseases (celiac disease, IBS), supporting a systemic epithelial barrier hypothesis.
– One study estimated adjusted odds ratios (ORs) for multiple EBDs in patients with SD; the other reported bidirectional hazard ratios (HRs) showing increased risk both of developing SD after an EBD and developing EBDs after SD.
Background
Seborrheic dermatitis (SD) is a common chronic inflammatory disorder predominantly affecting sebaceous-rich sites (scalp, face, chest) and characterized clinically by erythema and flaking. Beyond local symptoms, recent conceptual frameworks — notably the epithelial barrier theory (EBT) — propose that epithelial barrier disruption is a unifying pathogenic mechanism across diverse organ systems. Under EBT, barrier dysfunction (impaired tight junctions, lipid disorganization, dysregulated antimicrobial peptides, and microbiome alterations) promotes local inflammation and may predispose to immunologic and microbial perturbations at distal epithelial surfaces. Clarifying SD’s associations with other epithelial barrier diseases (EBDs) has implications for screening, pathogenesis research, and therapeutic strategies focused on barrier restoration.
Study designs
Study 1 — Epithelial Barrier Diseases Among Adult Patients With Seborrheic Dermatitis (JAMA Dermatol., 2025)
Design: Retrospective cohort analysis of a large US administrative claims database from January 1, 2016, through June 30, 2022.
Population: 20,274,189 adults (≥18 years) with ≥1 year of continuous enrollment and ≥2 distinct medical visits. Median age among SD patients was 62.6 years; 54.7% female. Total follow-up exceeded 70 million person-years (mean follow-up 3.46 ± 1.80 years).
Exposure/outcome: Exposure was diagnosis of SD during observation; primary outcomes were diagnoses of various EBDs during the observation period.
Analysis: Adjusted models (multivariable) evaluated the association between SD and subsequent or concurrent diagnoses of multiple EBDs, reported as odds ratios (ORs) with 95% confidence intervals (CIs).
Study 2 — Bidirectional Associations Between SD and EBDs (Allergy, 2025)
Design: Retrospective cohort study using a similarly large US administrative claims database (January 1, 2016–June 30, 2022).
Population: 5,083,689 patients with mean follow-up 3.25 ± 1.75 years and total follow-up >16 million person-years.
Analysis: The authors examined bidirectional temporal associations: risk of SD after an index EBD, and risk of an EBD after an index SD, using multivariable Cox proportional hazards models reporting hazard ratios (HRs) with 95% CIs.
Key findings and comparative results
Both studies report consistent positive associations between SD and numerous EBDs; the magnitude and temporal framing differ (ORs in Study 1 vs HRs in Study 2), but the directionality and affected disease domains overlap considerably.
Skin disease comorbidities
Study 1 (adjusted ORs): atopic dermatitis OR 3.21 (95% CI 3.18–3.24); psoriasis OR 3.26 (3.23–3.29); alopecia areata OR 4.02 (3.93–4.11); rosacea OR 4.52 (4.49–4.56); contact dermatitis OR 2.25 (2.23–2.26); hidradenitis suppurativa OR 1.63 (1.58–1.68).
Study 2 (bidirectional HRs): risk of SD after atopic dermatitis HR 2.46 (2.40–2.53), alopecia areata HR 3.47 (3.24–3.71), contact dermatitis HR 1.92 (1.88–1.96), psoriasis HR 2.62 (2.54–2.69), rosacea HR 2.84 (2.78–2.90), hidradenitis suppurativa HR 1.79 (1.63–1.97). In the reverse direction (EBD after SD), the largest HRs were psoriasis 3.52 (3.42–3.61), rosacea 2.85 (2.79–2.92), and alopecia areata 2.81 (2.61–3.03).
Interpretation: Both studies show strong associations (often >2-fold) between SD and inflammatory skin diseases characterized by barrier impairment and immune dysregulation. The highest effect sizes are seen for alopecia areata, psoriasis, and rosacea.
Non-cutaneous epithelial associations
Study 1 reported positive associations between SD and several non-cutaneous EBDs: rhinosinusitis OR 1.24 (1.24–1.25); celiac disease OR 1.36 (1.32–1.39); irritable bowel syndrome (IBS) OR 1.32 (1.31–1.33); ocular allergy OR 1.39 (1.37–1.41); dry eye OR 1.48 (1.48–1.49). Notably, SD was negatively associated with COPD OR 0.72 (0.71–0.72) and pulmonary hypertension OR 0.70 (0.69–0.71).
Study 2 also identified increased risk of SD after rhinosinusitis HR 1.34 (1.32–1.35), food allergy HR 1.47 (1.42–1.54), celiac disease HR 1.55 (1.43–1.68), ocular allergy HR 1.55 (1.49–1.61), and dry eye HR 1.54 (1.52–1.56). The reverse direction (EBD after SD) showed similar trends.
Interpretation: Both datasets support cross‑system associations involving respiratory, ocular, and gastrointestinal epithelia. Effect sizes are more modest than for dermatologic comorbidities but are consistent and statistically robust given sample sizes.
Bidirectionality and temporality
Study 2 explicitly evaluated bidirectionality and found that risks exist in both temporal directions: an EBD increases the hazard of developing SD and an index SD increases the hazard of developing other EBDs. This bidirectional pattern strengthens causal plausibility that shared mechanisms (e.g., systemic epithelial vulnerability or shared environmental exposures) contribute to comorbidity, while acknowledging that claims data cannot definitively prove causation.
Biological plausibility and mechanistic insights
These epidemiologic signals align with mechanistic aspects of EBT: barrier disruption (epidermal and mucosal) can permit enhanced antigen penetration, dysregulated innate immune signaling, altered lipid barrier composition, and microbiome shifts. In SD specifically, sebum-rich environments, Malassezia-dominated microbiota, and local immune activation contribute to barrier dysfunction. It is plausible that genetic predisposition, environmental exposures (pollutants, detergents), and systemic immune milieu create a milieu predisposing multiple epithelial surfaces to disease manifestations. However, direct mechanistic links (e.g., shared biomarkers, permeability measurements, or causal microbial translocation) remain to be demonstrated.
Expert commentary, limitations, and generalizability
Strengths of the studies include very large sample sizes, diverse clinical settings captured in administrative claims, and consistent patterns across independent analyses. Study 1 benefits from the very large denominator (over 20 million), yielding precise effect estimates; Study 2 adds value by assessing temporality and bidirectionality.
Key limitations should temper interpretation:
- Data source: Administrative claims are optimized for billing, not clinical phenotyping. Diagnostic misclassification and variable coding sensitivity/specificity are inherent risks.
- Residual confounding: Although multivariable adjustment was used, unmeasured confounders (smoking, socioeconomic status, obesity, medication use such as systemic immunosuppressants) could influence associations.
- Ascertainment bias: Patients with one chronic condition may have higher healthcare utilization and consequently higher probability of additional diagnoses.
- Temporality and causality: Even with bidirectional Cox models, observational claims data cannot establish causation or the mechanistic sequence of barrier disruption leading to systemic disease.
- Population representativeness: The studies rely on insured populations in the US; results may not generalize to uninsured populations or other countries with different healthcare systems or prevalence patterns.
Clinical implications
For clinicians, these results underscore that SD is not merely a localized cosmetic or nuisance condition; it associates with a wide range of dermatologic and non-dermatologic EBDs. Practical clinical implications include heightened vigilance for comorbid conditions (dry eye symptoms, chronic rhinosinusitis, celiac disease symptoms, food allergy history) and integrated care pathways: dermatologists might liaise with allergists/immunologists, gastroenterologists, and ophthalmologists when patients present with multi‑system symptoms.
Therapeutically, the findings support rationale for barrier-focused interventions (emollients, gentle cleansers, agents that restore lipids/tight junction integrity) as potential adjuncts to disease-targeted therapies. However, interventional trials would be required to demonstrate that barrier restoration in SD reduces incidence or severity of other EBDs.
Research gaps and future directions
Priority research steps include:
- Prospective cohorts with standardized phenotyping, biomarker panels (barrier function assays, cytokine profiles), and microbiome sampling to map temporal and mechanistic relationships.
- Interventional studies testing whether barrier-strengthening therapies in SD reduce risk or severity of comorbid EBDs.
- Genetic and transcriptomic studies to identify shared susceptibility pathways across epithelial surfaces.
- Investigations into why some associations are negative (e.g., COPD) to clarify confounding or protective mechanisms.
Conclusion
Two complementary large retrospective studies provide convergent epidemiologic evidence that seborrheic dermatitis is associated with increased prevalence and incidence of multiple epithelial barrier diseases across organ systems, with bidirectional temporal relationships. These observations lend population-level support to the epithelial barrier theory as a shared contributor to multi-system disease. Clinicians should be attentive to possible comorbid EBDs in patients with SD, and researchers should prioritize mechanistic and interventional studies to test whether barrier restoration can modify risk.
Funding and clinicaltrials.gov
Funding and specific trial registrations are reported in the original publications (see References). Both studies were retrospective analyses of administrative claims data and did not report clinicaltrial.gov identifiers.
References
1. Meng S, Berna R, Hoffstad O, Takeshita J, Shin D, Chiesa Fuxench ZC, Margolis DJ. Epithelial Barrier Diseases Among Adult Patients With Seborrheic Dermatitis. JAMA Dermatol. 2025 Nov 5:e254313. doi: 10.1001/jamadermatol.2025.4313. Epub ahead of print. PMID: 41191376; PMCID: PMC12590386.
2. Meng S, Berna R, Takeshita J, Hoffstad O, Shin D, Mitra N, Margolis DJ. Bidirectional Associations Between Seborrheic Dermatitis and Epithelial Barrier Diseases: A Retrospective Cohort Study. Allergy. 2025 Oct 27. doi: 10.1111/all.70112. Epub ahead of print. PMID: 41144761.
AI image prompt for article thumbnail
Close-up clinical montage: a dermatologist examining a patient’s scalp with visible seborrheic dermatitis flakes; schematic overlay of an epithelial barrier (tight junction proteins, lipid layers) with faint icons representing gut, eye, and sinuses; soft clinical lighting, muted blues and greens, realistic photographic style, high detail.