The Dawn of Restorative Vision: Addressing the Challenge of Geographic Atrophy
Geographic atrophy (GA) represents the advanced, non-exudative stage of age-related macular degeneration (AMD), characterized by the progressive and irreversible loss of retinal pigment epithelium (RPE), photoreceptors, and the underlying choriocapillaris. For decades, GA has been synonymous with a steady decline in central vision, leading to profound disability in tasks such as reading, recognizing faces, and driving. With over 5 million people affected worldwide, the medical community has long sought a solution that moves beyond slowing progression to actually restoring lost functional vision. The publication of the PRIMAvera study results in the New England Journal of Medicine marks a transformative moment in this quest, introducing a subretinal photovoltaic system designed to bypass damaged photoreceptors and directly stimulate the remaining inner retinal layers.
The PRIMA System: A Fusion of Photovoltaics and Neural Engineering
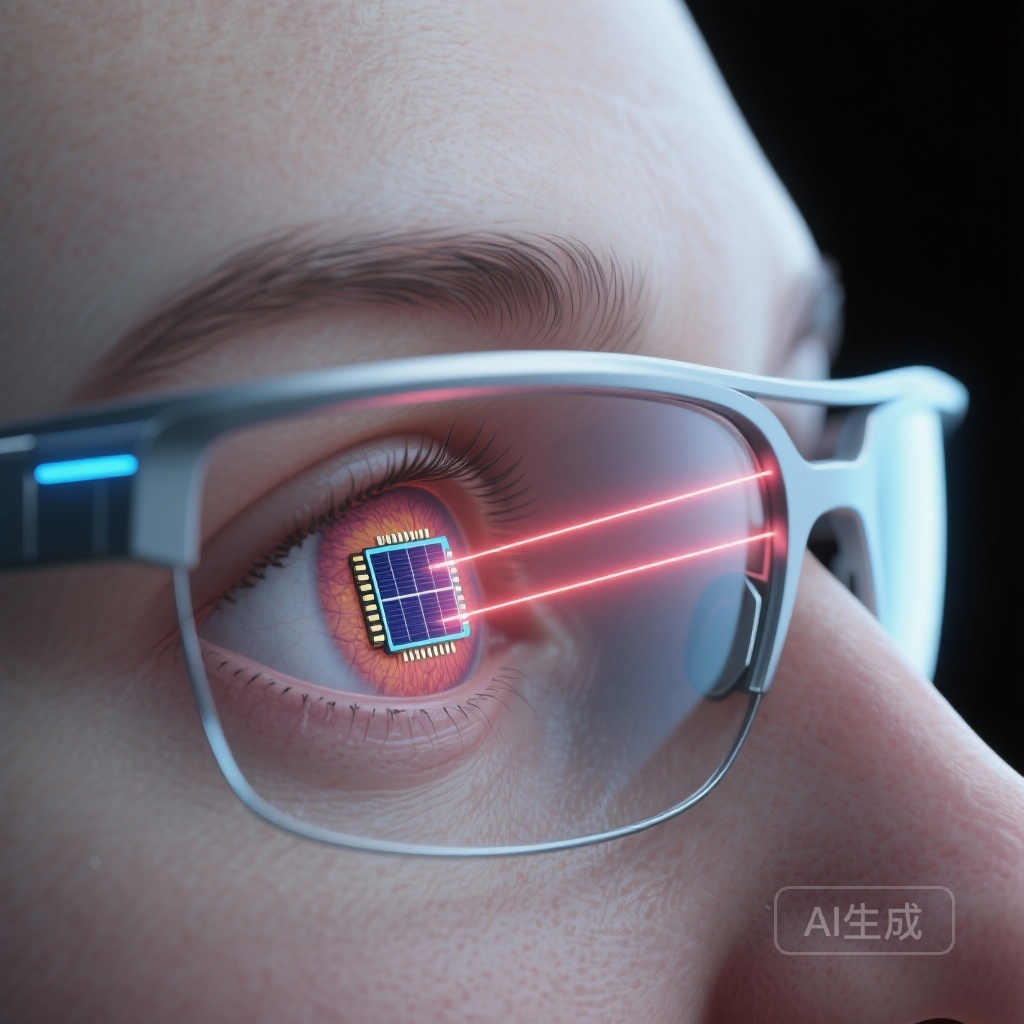
The Photovoltaic Retina Implant Microarray (PRIMA) system is a sophisticated bionic vision platform. Unlike earlier epiretinal implants that required complex internal wiring and bulky external hardware, the PRIMA system utilizes a wireless, subretinal approach. The system consists of three primary components: a miniaturized subretinal implant, a pair of augmented reality glasses equipped with a camera, and a pocket-sized processor.
Mechanism of Action
The implant itself is a 2-mm wide, 30-micron thick silicon chip containing 378 hexagonal pixels. Each pixel functions as an independent photovoltaic cell. The external glasses capture visual information from the environment and project it as near-infrared (880 nm) light patterns onto the macula. The subretinal chip then converts this light into electrical currents, which stimulate the surviving bipolar cells of the inner retina. By leveraging the eye’s existing neural architecture—specifically the second- and third-order neurons—the system aims to restore a form of artificial central vision that the brain can interpret as visual images.
The PRIMAvera Study: Clinical Trial Design and Methodology
The PRIMAvera study (NCT04676854) was an open-label, multicenter, prospective, single-group, baseline-controlled clinical trial. The study enrolled 38 participants suffering from geographic atrophy due to AMD. Inclusion criteria required a baseline visual acuity of at least 1.2 logMAR (approximately 20/320 or worse) in the study eye, ensuring that the intervention was targeted at those with severe central vision loss.
Study Endpoints
The primary efficacy endpoint was a clinically meaningful improvement in visual acuity, defined as a gain of 0.2 logMAR (equivalent to 2 lines on a standard Snellen chart) or more from baseline at 12 months post-implantation. The primary safety endpoint focused on the incidence and severity of serious adverse events (SAEs) related to either the surgical procedure or the device itself throughout the 12-month follow-up period.
Key Findings: Significant Improvements in Visual Acuity
The results of the PRIMAvera trial provide robust evidence for the efficacy of the subretinal photovoltaic system. Of the 38 participants who received the implant, 32 were available for assessment at the 12-month mark. The remaining six participants were accounted for through multiple imputation models to ensure the integrity of the statistical analysis.
Efficacy Data
Among the 32 participants who completed the 12-month follow-up, 26 (81%) achieved a clinically meaningful improvement in visual acuity of 0.2 logMAR or greater. The 95% confidence interval (CI) for this outcome ranged from 64% to 93%, with a highly significant p-value of <0.001. When using multiple imputation to include the missing data from all 38 participants, the estimated success rate remained high at 80% (95% CI, 66 to 94; P<0.001). This level of improvement is unprecedented in a population where vision loss was previously considered permanent.
Peripheral Vision Preservation
A critical concern with subretinal implants is the potential for surgical trauma or the device itself to damage the remaining natural peripheral vision. The PRIMAvera study found that the mean natural peripheral visual acuity after implantation remained equivalent to baseline levels. This suggests that the PRIMA system can restore central vision without compromising the patient’s existing ambulatory peripheral vision, a vital factor for patient safety and mobility.
Safety and Tolerability Profile
The safety profile of the PRIMA system was carefully monitored. A total of 26 serious adverse events occurred in 19 participants. The majority of these events (81%) were concentrated within the first two months following the surgical procedure, indicating that most risks are associated with the perioperative phase rather than long-term device presence. Notably, 95% of these early SAEs resolved within two months of onset. Common events included typical surgical complications such as subretinal hemorrhage or transient intraocular pressure elevations, which were manageable with standard ophthalmic care.
Expert Commentary: Clinical Implications and Future Directions
The success of the PRIMA system represents a paradigm shift in the management of geographic atrophy. While recent pharmacological advancements (such as complement inhibitors) focus on slowing the expansion of GA lesions, they do not restore lost vision. The PRIMA system addresses the structural deficit by replacing lost photoreceptors with silicon photodiodes.
Biological Plausibility and Integration
The ability of the brain to adapt to artificial electrical signals from the retina—often referred to as neuroplasticity—is central to the success of this technology. Participants in the study underwent visual rehabilitation to learn how to interpret the new signals. The high rate of success suggests that the remaining retinal circuitry in GA patients is sufficiently intact to transmit meaningful visual information to the cortex.
Study Limitations
While the results are promising, clinicians must consider the limitations. This was a single-group, baseline-controlled study rather than a randomized controlled trial. Additionally, the surgical procedure requires specialized vitreoretinal expertise. Future research will likely focus on optimizing the resolution of the chip (increasing pixel density) and expanding the indications for other forms of outer retinal degeneration.
Conclusion
The PRIMAvera study demonstrates that the subretinal photovoltaic implant is a safe and effective means of restoring central vision in patients with advanced geographic atrophy. With 80% of participants achieving a significant gain in visual acuity and the preservation of peripheral vision, this technology offers a new horizon for millions of patients currently living with the consequences of AMD. As the technology matures, it may become a standard of care for restoring sight in the blind.
Funding and ClinicalTrials.gov
This study was funded by Science Corporation and the Moorfields National Institute for Health and Care Research (NIHR) Biomedical Research Centre. The trial is registered at ClinicalTrials.gov under the number NCT04676854.
References
Holz FG, Le Mer Y, Muqit MMK, Hattenbach LO, Cusumano A, Grisanti S, Kodjikian L, Pileri MA, Matonti F, Souied E, Stanzel BV, Szurman P, Weber M, Bartz-Schmidt KU, Eter N, Delyfer MN, Girmens JF, van Overdam KA, Wolf A, Hornig R, Corazzol M, Brodie F, Olmos de Koo L, Palanker D, Sahel JA. Subretinal Photovoltaic Implant to Restore Vision in Geographic Atrophy Due to AMD. N Engl J Med. 2026 Jan 15;394(3):232-242. doi: 10.1056/NEJMoa2501396. Epub 2025 Oct 20. PMID: 41124203.