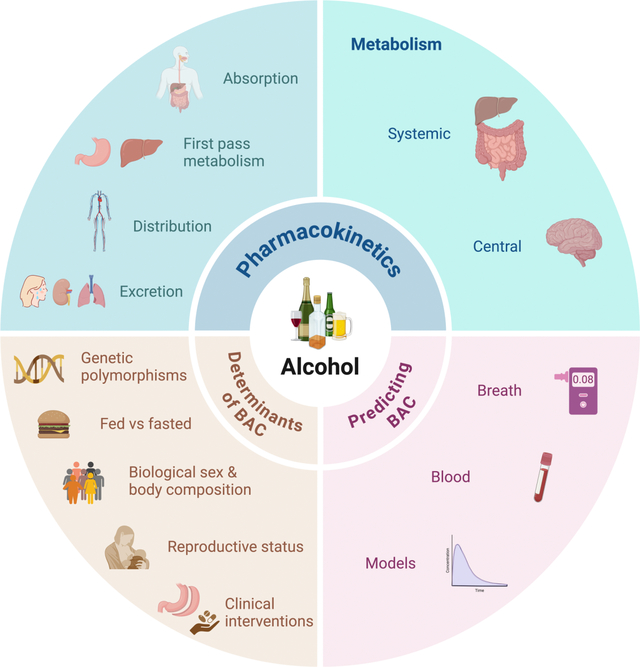
Alcohol remains the most widely consumed psychoactive substance globally and a major contributor to premature mortality, especially among individuals aged 15 to 49 years. Understanding the pharmacokinetics of alcohol—including its absorption, distribution, metabolism, and excretion—is pivotal for predicting its behavioral effects and toxicity. Over recent decades, research has advanced our knowledge of alcohol metabolism not only in the liver and gut but also centrally within the brain. Moreover, variability in this metabolic processing, influenced by genetic polymorphisms, sex, physiological states such as pregnancy and lactation, and clinical interventions such as bariatric surgery, has significant implications for individual susceptibility to alcohol use disorder (AUD) and alcohol-related diseases.
Evolutionary and Historical Context of Alcohol Metabolism
Alcohol metabolism enzymes, particularly alcohol dehydrogenases (ADHs), evolved over 400 million years ago, predating natural exposure to ethanol. In vertebrates, type I ADHs predominate and encompass multiple isoenzymes with diverse catalytic efficiencies and tissue-specific expression patterns. A notable mutation in the ADH7 gene, producing ADH4, occurred approximately 10 million years ago in hominids, increasing enzymatic capacity to metabolize ethanol and possibly adapting primates to terrestrial consumption of fermenting fruits.
Historically, alcohol has played dual roles culturally and medicinally. Ancient societies prized alcoholic beverages for nutrition, social rituals, and therapeutic uses, sometimes disseminating myths about its benefits, such as facilitating lactation, despite contrary scientific evidence.
Alcohol Absorption and First-Pass Metabolism
Alcohol is absorbed primarily in the small intestine via passive diffusion, with gastric emptying rate being a key determinant of systemic absorption. Carbonation accelerates absorption, and food intake delays it by slowing gastric emptying and increasing first-pass metabolism (FPM).
FPM refers to metabolism occurring in the gastrointestinal tract and liver before systemic circulation. The stomach, especially via gastric ADH class IV (ADH4), contributes significantly to FPM, as indicated by evidence showing reduced FPM after gastrectomy and with enzyme inhibition (e.g., cimetidine). Hepatic metabolism also participates in FPM but is influenced by alcohol delivery rate, which depends on gastric emptying. Bariatric surgeries that alter gastric anatomy and emptying, such as sleeve gastrectomy (SG) and Roux-en-Y gastric bypass (RYGB), reduce gastric FPM, resulting in elevated alcohol bioavailability and faster peak blood alcohol concentrations (BACs).
Table 1.
Human alcohol dehydrogenase (ADH)
| ADH Class | Official Gene Nomenclature | Former Gene Nomenclature | Subunit Nomenclature | Enzyme Nomenclature | Km* | Vmax* | Tissue expression |
|---|---|---|---|---|---|---|---|
| I | ADH1A | ADH1 | α | ADH1A | 4 | 30 | Liver |
| ADH1B*1 | ADH2*1 | β1 | ADH1B | 0.013 | 5.2 | Liver, adipose, brain, breast | |
| ADH1B*2 | ADH2*2 | β2 | ADH1B | 1.8 | 190 | ||
| ADH1B*3 | ADH2*3 | β3 | ADH1B | 61 | 140 | ||
| ADH1C*1 | ADH3*1 | γ1 | ADH1C | 0.1 | 32 | Liver, stomach, intestine | |
| ADH1C*2 | ADH3*2 | β2 | ADH1C | 0.14 | 20 | ||
| II | ADH4 | ADH4 | π | ADH2 | 11 | 9 | Liver |
| III | ADH5 | ADH5 | χ | ADH3 | >1000 | 100 | Most tissues |
| IV | ADH7 | ADH7 | σ (μ) | ADH4 | 30 | 1800 | Stomach, esophagus, mucosae |
| V | ADH6 | ADH6 | Not identified | ADH5 | ? | ? | Liver, stomach |
Distribution and Elimination of Alcohol
Once absorbed, ethanol distributes according to total body water, and because it poorly partitions into adipose tissue, individuals with higher fat content—such as women and the elderly—tend to have higher peak BACs given equal doses. Ethanol elimination is predominantly hepatic, accounting for 94–98% of its clearance via oxidative metabolism, primarily by ADHs converting ethanol to acetaldehyde, subsequently metabolized by aldehyde dehydrogenases (ALDHs) to acetate.
Figure 1. Modulators of first-pass metabolism (FPM) and their impact on ethanol bioavailability.

Secondary pathways include the microsomal ethanol-oxidizing system (MEOS), involved notably at high alcohol concentrations or chronic exposure, and catalase, which plays a minor role physiologically. Non-oxidative metabolism generates metabolites such as ethyl glucuronide (EtG), ethyl sulfate (EtS), fatty acid ethyl esters (FAEEs), and phosphatidylethanol (PEth), which serve as biomarkers of recent alcohol consumption and may contribute to tissue injury.
Figure 2. Pathways of ethanol elimination in the human body.

Figure 3. Oxidative pathways of hepatic ethanol metabolism.
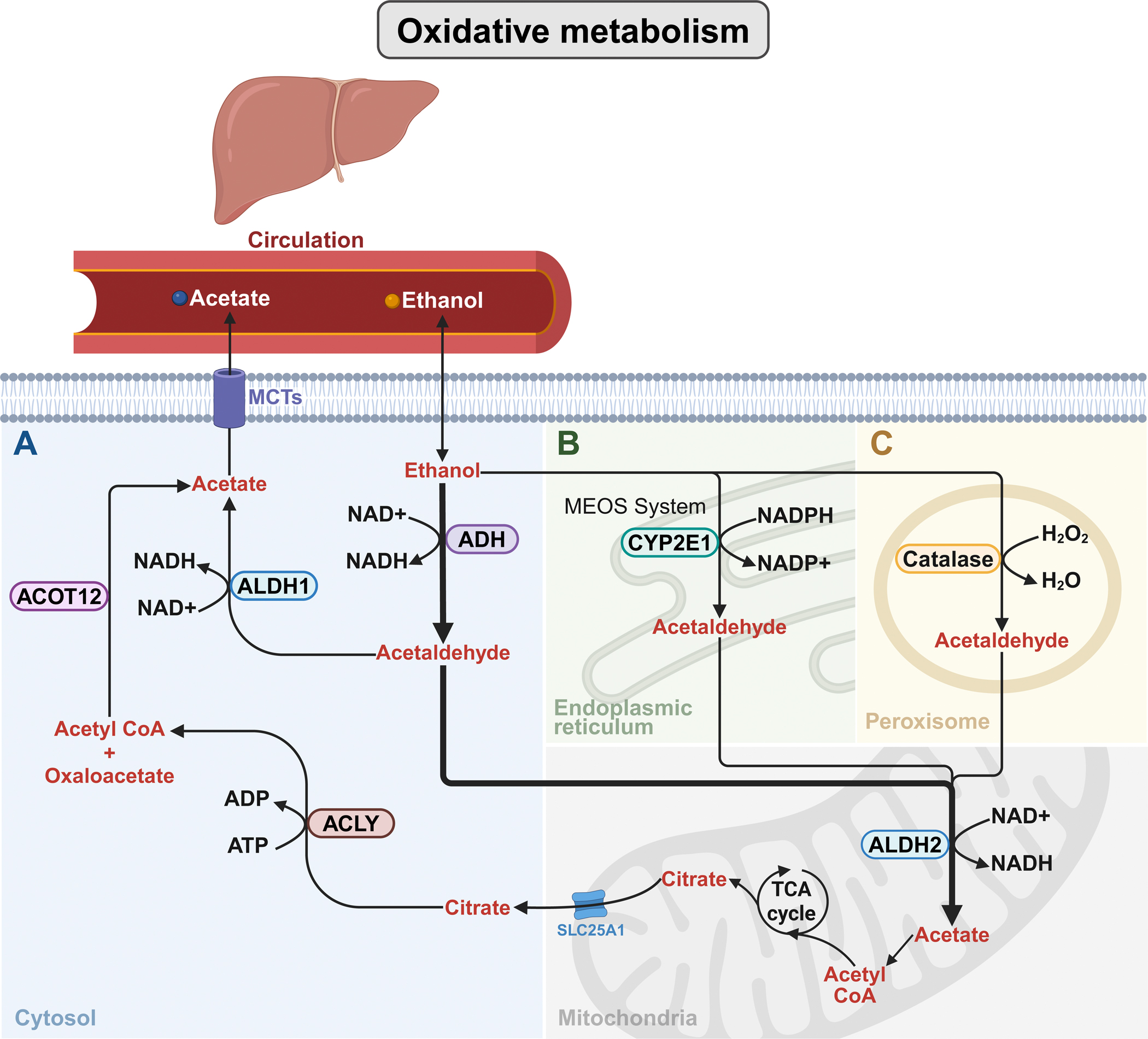
Figure 4. Non-oxidative metabolism of alcohol.

Central (Brain) Alcohol Metabolism
Brain metabolism of ethanol uses similar enzymatic systems but prioritizes catalase over ADH for oxidizing ethanol to acetaldehyde. CYP2E1 is also expressed and inducible in brain regions implicated in alcohol’s reinforcing effects.
Figure 5. Brain metabolism of ethanol.
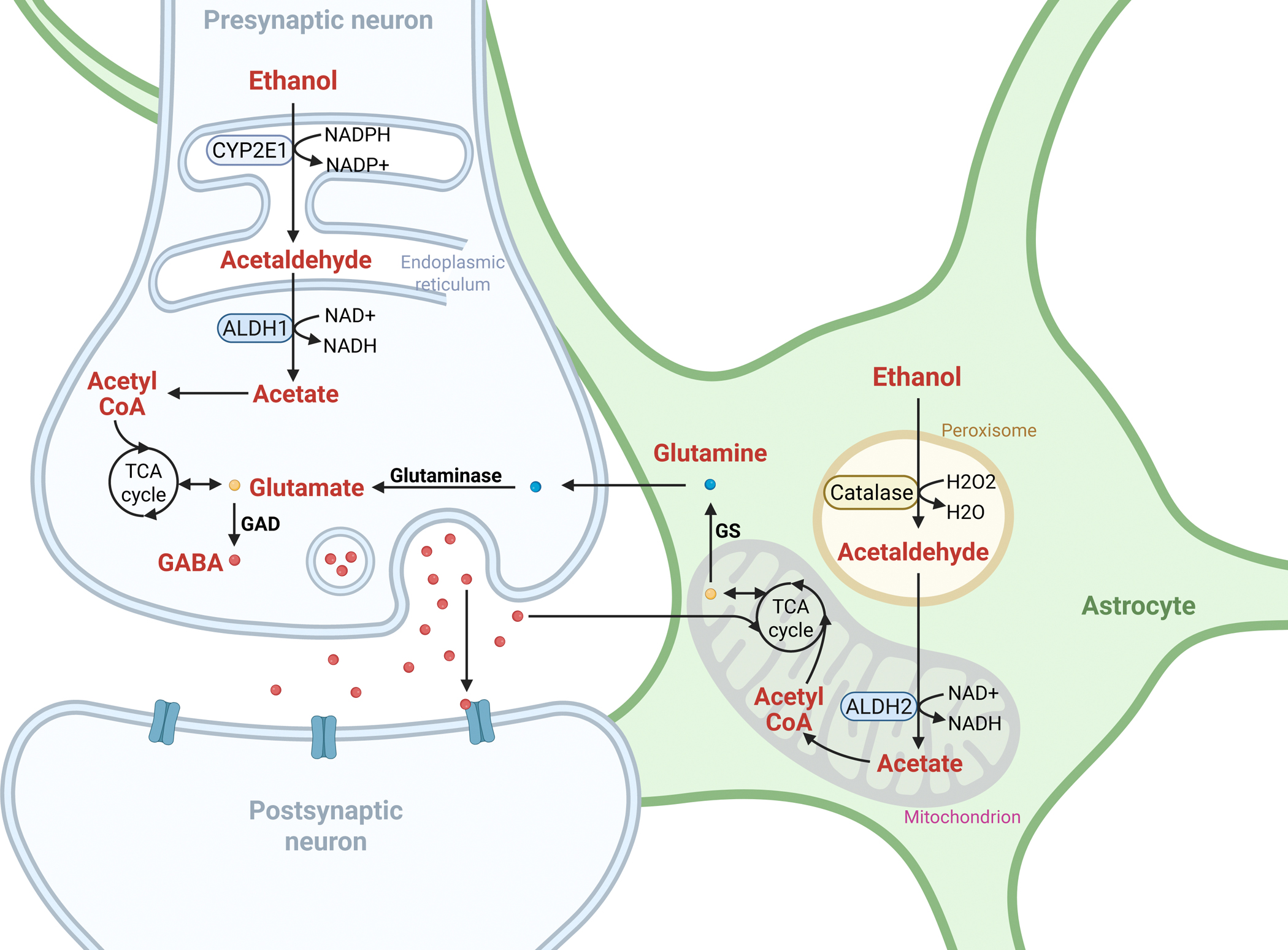
Acetaldehyde and acetate produced in the brain influence neurotransmitter systems, especially GABAergic neurotransmission, contributing to alcohol’s sedative and intoxicating effects. Experimental manipulations suggest brain-derived acetaldehyde may mediate early reinforcing responses to alcohol, though its role in chronic consumption is less clear. Notably, regional differences in ALDH2 expression, such as high cerebellar astrocytic ALDH2, modulate alcohol’s neuromotor effects.
Non-oxidative metabolism also occurs in the brain, with enzymatic synthesis of FAEEs potentially contributing to neurotoxicity.
Factors Affecting Alcohol Metabolism
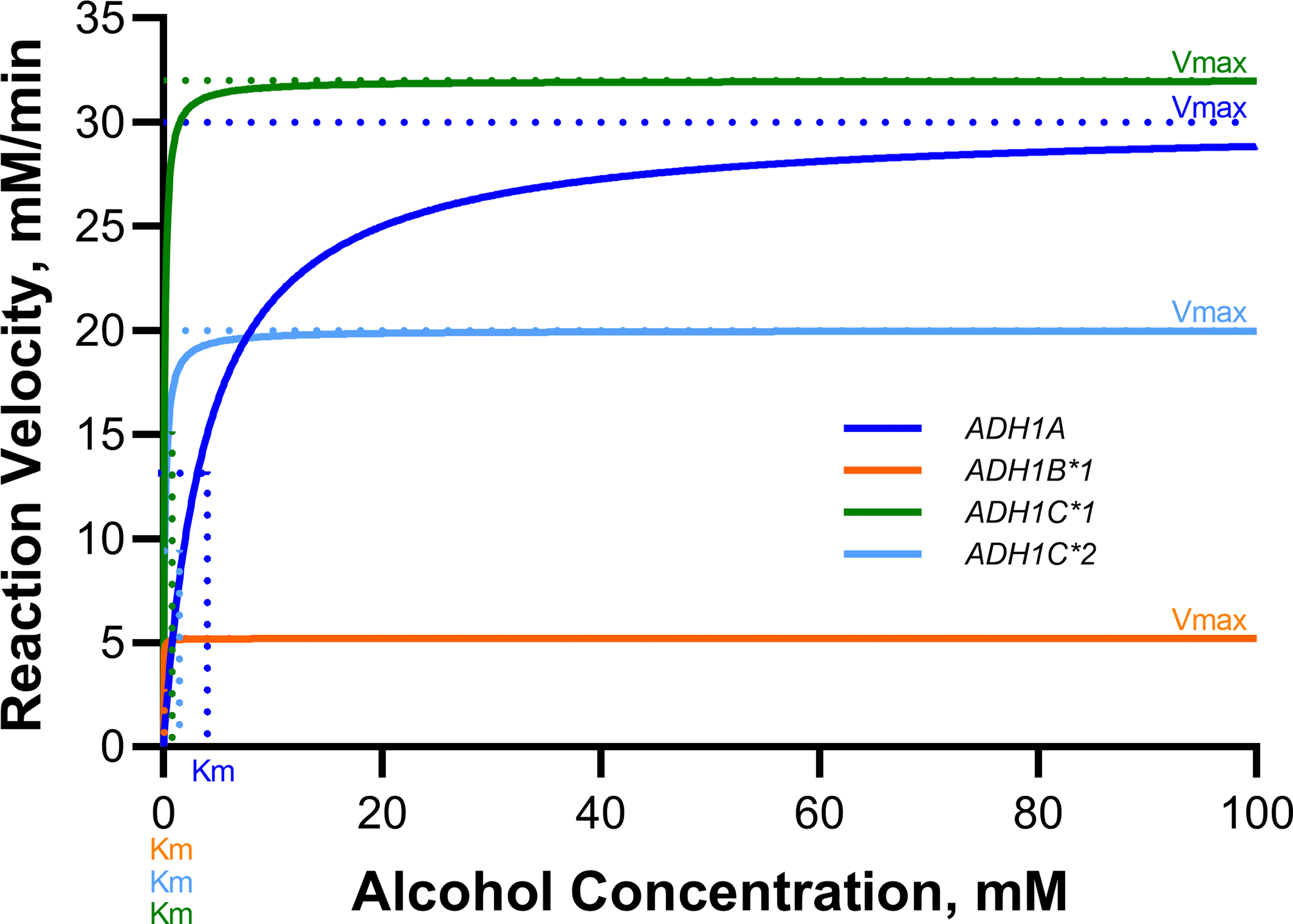
Genetic polymorphisms in ADH and ALDH genes dramatically affect enzymatic activity and thus alcohol metabolism rates. For example, the ADH1B*2 allele common in East Asians encodes a highly active enzyme accelerating ethanol oxidation, while the ALDH2*2 variant impairs acetaldehyde metabolism leading to acetaldehyde accumulation, aversive reactions, and a reduced risk of AUD but elevated cancer risk.
Figure 6. Enzymatic activity for common isoforms of alcohol dehydrogenase (ADH).
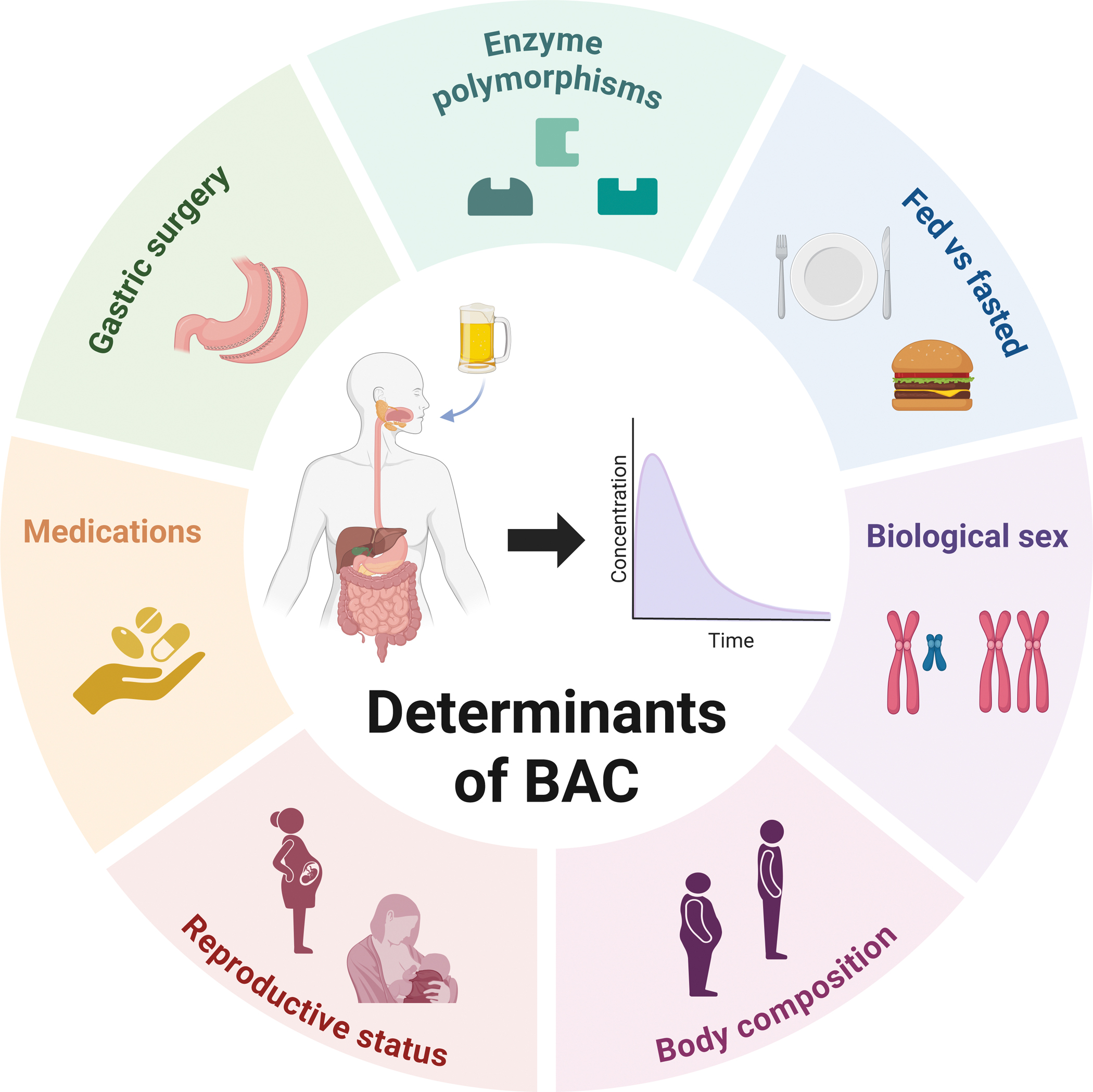
Nutritional status modulates alcohol pharmacokinetics; food intake delays absorption and increases both first-pass metabolism and systemic elimination rates, reducing peak BACs.
Biological sex differences arise from body composition—females generally have higher fat-to-lean ratios and lower gastric ADH activity, leading to higher peak BACs.
Pregnancy and lactation modulate alcohol pharmacokinetics through altered volume of distribution and increased first-pass metabolism, with lactation also involving hormonal influences on gastric motility.
Figure 7. Determinants of blood alcohol concentration (BAC).
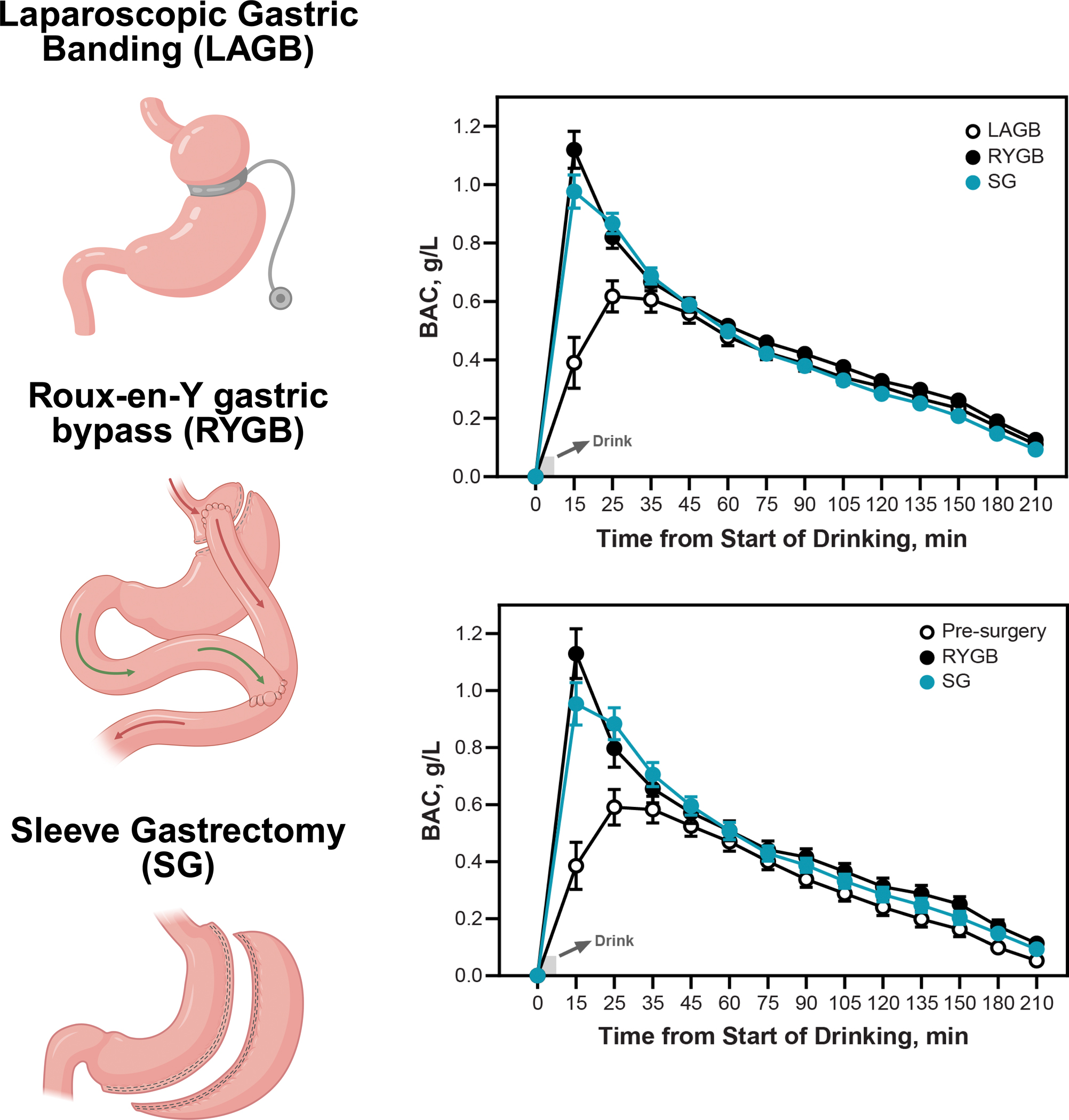
Bariatric surgeries such as RYGB and SG reduce gastric FPM and increase peak BACs after alcohol consumption, correlating with an elevated risk of AUD and alcohol-associated liver disease postoperatively.
Many medications interact with alcohol metabolism either by competing for metabolic enzymes (e.g., CYP450 isoforms) or altering absorption, exemplified by benzodiazepines, opioids, antibiotics, NSAIDs, and acetaminophen, raising safety concerns.
Clinical Implications and Therapeutic Approaches
Drugs targeting ethanol metabolism remain important in AUD treatment. Disulfiram irreversibly inhibits ALDH, inducing aversive reactions to alcohol. Cyanamide similarly inhibits ALDH but is less widely used due to hepatotoxicity. Metadoxine accelerates alcohol clearance and shows promise in AUD management but requires further clinical validation. Fomepizole, an ADH inhibitor approved for toxic alcohol poisonings, reduces alcohol consumption in animal models but carries potential toxicity risks due to diversion to non-oxidative pathways.
Emerging treatments targeting ALDH2 and RNA interference approaches are under investigation but require cautious evaluation due to safety concerns.
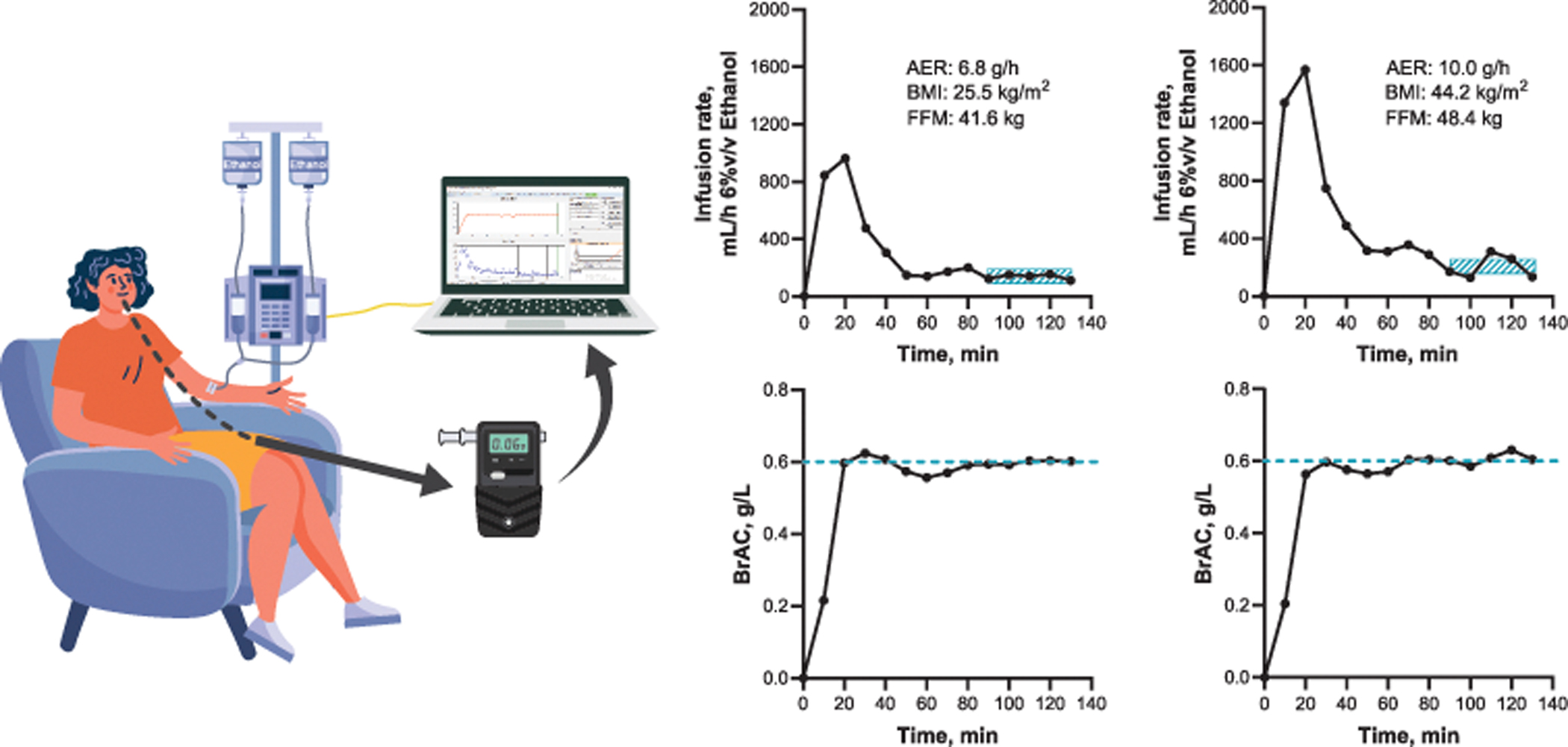
Figure 8. Impact of body composition on alcohol elimination rates (AER) using the breath alcohol clamping technique.
Conclusions and Future Directions
Recent advances underscore alcohol metabolism as a complex, multi-organ process extending beyond hepatic pathways to include significant brain metabolism and gut-liver axis interplay. Genetic, physiological, and clinical factors contribute to wide interindividual variability with important implications for addiction risk, toxicity, and therapeutic strategies.
Figure 10. Impact of gastric surgeries on blood alcohol concentration (BAC).
Understanding the gut-liver axis in alcohol detoxification, particularly the role of bile acid dynamics, presents opportunities for new interventions. The growing recognition of endogenous ethanol production by gut microbiota links alcohol metabolism to metabolic liver disease and necessitates deeper investigation.
Advancing brain-specific metabolic studies using neuroimaging and molecular techniques promises novel insights into alcohol’s neurological effects and individual vulnerability.
Furthermore, the implications of bariatric surgery on alcohol pharmacokinetics require enhanced clinical vigilance and patient education to mitigate long-term risks.
Finally, integrating alcohol metabolism considerations into medication management is critical to preventing adverse interactions and optimizing treatment outcomes.
Continued translational research is essential to bridge existing knowledge gaps, inform personalized medicine approaches, and improve clinical care related to alcohol use and its disorders.
Reference:
Goldman MR, Molina-Castro M, Etkins JC, Koide TL, Ramchandani VA, Plawecki MH, Mennella JA, Pepino MY. Recent advances in alcohol metabolism: from the gut to the brain. Physiol Rev. 2025 Oct 1;105(4):2501-2535. doi: 10.1152/physrev.00053.2024 IF: 28.7 Q1 . Epub 2025 Jul 10. PMID: 40637545 IF: 28.7 Q1 ; PMCID: PMC12345593 IF: 28.7 Q1 .