High-Risk Pure Aortic Regurgitation: The Unmet Need
Pure native aortic regurgitation (AR) has long been considered the last frontier for transcatheter aortic valve replacement (TAVR). While TAVR has revolutionized the management of aortic stenosis, its application in pure AR remains technically challenging. The primary difficulty lies in the pathophysiology of the disease: unlike aortic stenosis, pure AR often presents with a lack of valvular calcification, an enlarged aortic annulus, and a dilated ascending aorta. These anatomical features provide insufficient anchoring for conventional TAVR devices, leading to high risks of valve migration, paravalvular leak, and the need for a second valve.
For patients with severe AR who are deemed high-risk or inoperable for traditional surgical aortic valve replacement (SAVR), the clinical outlook has historically been poor. Current guidelines emphasize the need for specialized devices specifically designed to address the unique anchoring requirements of non-calcified aortic valves. The Pioneer Trial, recently published in JACC: Cardiovascular Interventions, provides critical mid-term data on a novel system designed to bridge this therapeutic gap.
The Pioneer Valve: Engineering Solutions for Non-Calcified Anatomy
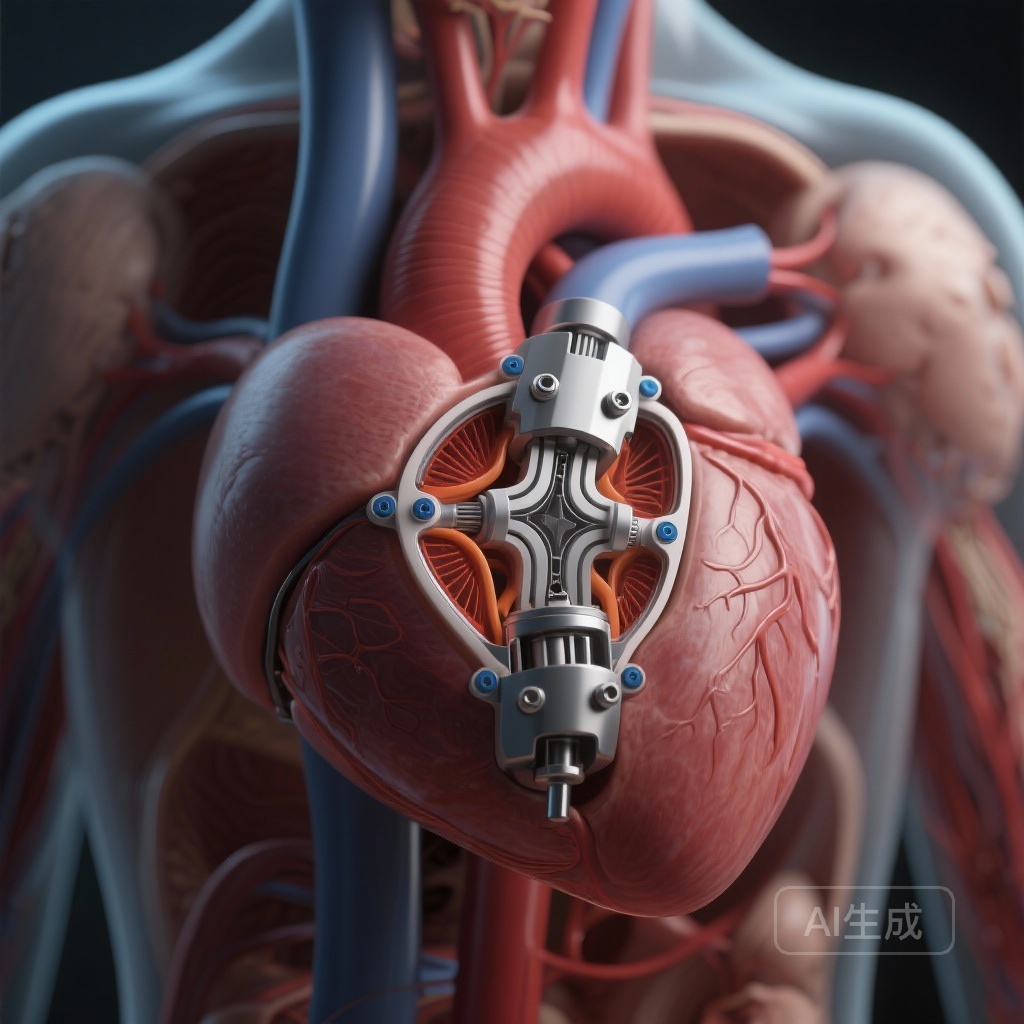
The Pioneer Valve system is a novel self-expanding, transfemoral TAVR device engineered specifically for pure native AR. Its design addresses the lack of calcium by incorporating a unique anchoring mechanism. The system features a rotatable delivery platform and three independently angle-adjustable locators. These locators are designed to clip onto the native aortic leaflets, providing stable fixation even in the absence of calcification. This mechanical ‘grasp’ mimics the surgical approach of securing a valve, significantly reducing the risk of device embolization or migration during deployment.
Study Design and Patient Population
The study was a prospective, single-arm, multicenter clinical trial (ChiCTR2300075152) conducted across 15 high-volume centers. A total of 110 consecutive patients with severe pure native AR were enrolled. The inclusion criteria targeted patients who were symptomatic and considered high-risk for conventional surgery, as determined by a multidisciplinary heart team.
The baseline characteristics of the cohort reflected a high-risk population. The mean age was 73.37 ± 4.21 years, and the median Society of Thoracic Surgeons (STS) score was 5.97%, with some patients reaching scores as high as 8.63%. All patients underwent rigorous pre-procedural imaging, including multidetector computed tomography (MDCT), to assess annular dimensions and suitability for the Pioneer system.
Key Findings: Technical Success and Survival
The primary focus of the trial was to evaluate technical success and mid-term safety. The results were highly encouraging, with technical success achieved in 109 out of 110 cases (99.1%). Only one instance of valve migration was reported, which is a significant improvement over historical outcomes using first-generation TAVR devices in off-label AR applications.
At the one-year follow-up mark (median duration of 348 days), the safety profile remained robust. The all-cause mortality rate was remarkably low at 2.7% (3 of 110 patients; 95% CI: 0.9%-8.3%). This survival rate is particularly notable given the high surgical risk profile of the participants. Furthermore, the incidence of major stroke and other VARC-3 (Valve Academic Research Consortium 3) defined complications remained within acceptable clinical thresholds.
Hemodynamic Performance and Ventricular Remodeling
Beyond safety, the study demonstrated excellent hemodynamic performance for the Pioneer Valve. At 30 days post-procedure, the mean aortic valve gradient was 7.88 ± 2.7 mm Hg, and the mean effective orifice area (EOA) was 2.2 ± 0.64 cm2. These metrics remained stable through the one-year follow-up, indicating consistent valve function without early signs of structural deterioration.
Perhaps most importantly for the patients’ quality of life, the study observed significant positive left ventricular (LV) remodeling. Chronic AR leads to volume overload, causing LV dilation and eventual heart failure. Following the Pioneer Valve implantation, there were measurable reductions in both left ventricular end-diastolic diameter (LVEDD) and end-systolic diameter (LVESD). These anatomical improvements correlated with significant functional gains:
Functional Recovery
Patients experienced a substantial improvement in New York Heart Association (NYHA) functional class. By one year, the majority of patients moved from Class III/IV to Class I/II. This was mirrored by a significant increase in the Kansas City Cardiomyopathy Questionnaire (KCCQ) scores, reflecting a marked enhancement in overall health status and daily functioning.
Safety Considerations: Pacemaker Implantation
One area of continued monitoring for the Pioneer Valve, as with many self-expanding TAVR platforms, is the rate of new permanent pacemaker implantation (PPI). In this trial, new PPI occurred in 23 of 105 at-risk patients (21.9%; 95% CI: 14.3%-29.8%). While this rate is comparable to other self-expanding devices used in the aortic position, it remains a focal point for future device iterations and procedural refinements. Clinicians must balance the benefit of effective AR resolution against the long-term implications of pacemaker dependency.
Expert Commentary: Clinical Implications
The Pioneer Trial represents a significant step forward in the transcatheter management of pure AR. For years, clinicians have struggled with the ‘off-label’ use of devices intended for stenosis, often resulting in suboptimal outcomes. The Pioneer system’s dedicated locator mechanism appears to solve the primary anatomical hurdle of anchoring in non-calcified zones.
However, experts note that while the mid-term results are promising, long-term durability data (beyond 5 years) will be essential, especially if TAVR is to be considered for younger patients with AR. Additionally, the 21.9% pacemaker rate suggests that further optimization of the implantation depth and device tension may be necessary. The successful deployment across 15 different centers suggests that the Pioneer Valve has a manageable learning curve, which is critical for widespread clinical adoption.
Conclusion
The prospective, multicenter Pioneer Trial confirms that the Pioneer Valve system is a safe and effective intervention for high-risk patients with severe pure native aortic regurgitation. With a 99.1% technical success rate, low mortality at one year, and evidence of significant reverse cardiac remodeling, this novel TAVR device provides a much-needed therapeutic alternative for a patient population that previously had few viable options. Future studies will likely focus on expanding these findings to a broader range of surgical risk profiles and assessing the long-term structural integrity of the valve.