Highlight
1. Oral semaglutide lowers major adverse cardiovascular events (MACE) risk by 14% in patients with type 2 diabetes and cardiovascular or kidney disease.
2. The cardiovascular benefit of oral semaglutide is consistent regardless of baseline or in-trial use of SGLT2 inhibitors.
3. Safety profiles of oral semaglutide with or without SGLT2 inhibitors were similar, supporting combined therapy safety.
4. Results affirm oral semaglutide as a valuable therapeutic option in high cardiovascular risk diabetes populations.
Study Background and Disease Burden
Type 2 diabetes mellitus (T2DM) is a chronic metabolic disorder with a high prevalence globally, closely linked to increased risk of atherosclerotic cardiovascular disease (ASCVD) and chronic kidney disease (CKD). Both ASCVD and CKD substantially contribute to morbidity and mortality in this population. Current therapeutic paradigms aim not only for glycemic control but also for cardiovascular risk reduction.
Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) and sodium-glucose cotransporter-2 inhibitors (SGLT2i) have individually demonstrated cardiovascular and renal protective effects in T2DM patients with or at high risk of such complications. However, clinical evidence regarding their combined use, particularly with oral semaglutide (the first oral GLP-1 RA), remained sparse. Understanding the effects of combination therapy on cardiovascular safety and efficacy is crucial for optimizing treatment strategies in this high-risk group.
Study Design
The SOUL trial (NCT03914326) was a double-blind, placebo-controlled, randomized, event-driven, superiority study including 9,650 participants aged 50 years or older with T2DM and established ASCVD and/or CKD. Inclusion criteria required glycated hemoglobin levels between 6.5% and 10%. Participants were randomized to receive either once-daily oral semaglutide (up to 14 mg) or placebo added to standard care.
A prespecified subgroup analysis stratified participants according to baseline use of SGLT2i and any use of SGLT2i during the trial. The primary endpoint was the time to first major adverse cardiovascular event (MACE), a composite of cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke. Safety assessments included incidence rates of serious adverse events and gastrointestinal disorders.
Key Findings
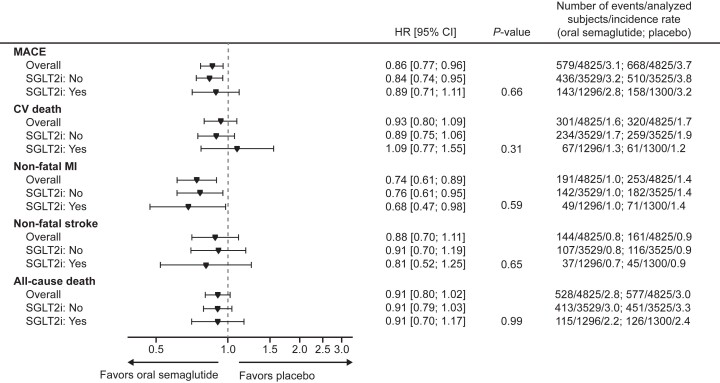
The median follow-up duration was approximately 49.5 months. Overall, oral semaglutide reduced the risk of the primary composite outcome by 14% compared to placebo (hazard ratio [HR]: 0.86, 95% confidence interval [CI]: 0.77–0.96; P=0.006). This translates clinically to fewer cardiovascular deaths, nonfatal myocardial infarctions, and strokes among treated patients.
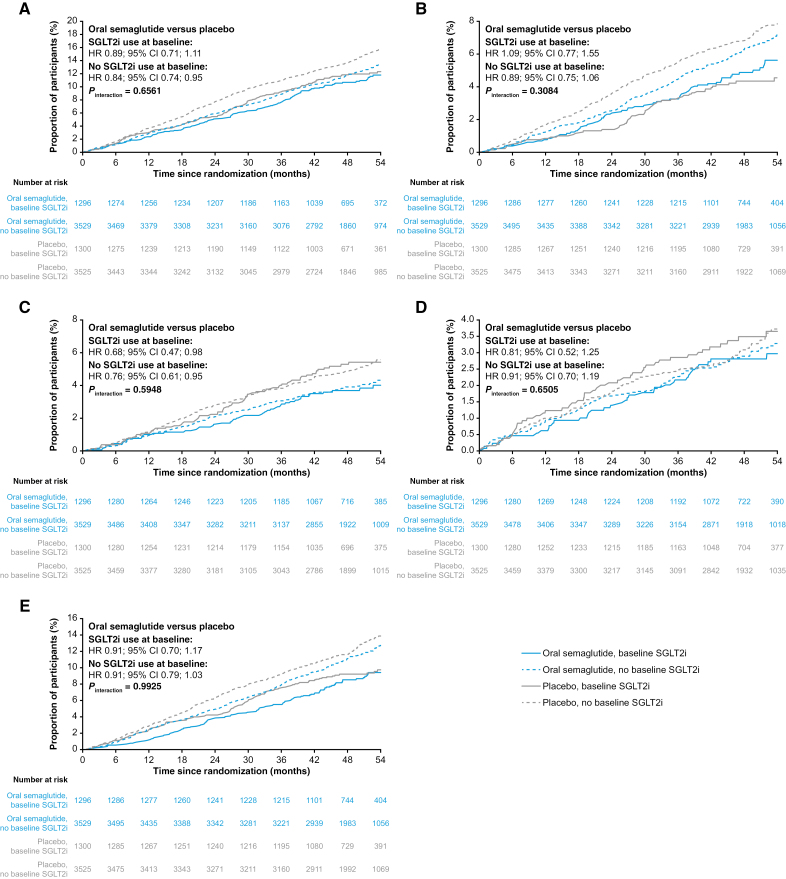
When stratified by baseline SGLT2i use, MACE event rates were similar across treatment groups: 143/1296 in the oral semaglutide group versus 158/1300 in placebo among SGLT2i users (HR 0.89; 95% CI, 0.71–1.11), and 436/3529 versus 510/3525 in non-users (HR 0.84; 95% CI, 0.74–0.95). The interaction test for treatment effect by baseline SGLT2i use showed no statistically significant heterogeneity (P-interaction = 0.66). Similarly, considering any in-trial SGLT2i use versus no use confirmed no differential effect.
Fig. Cumulative incidence plots of outcomes for oral semaglutide vs placebo in subgroups with or without SGLT2i use at baseline.
Forest plot: primary outcomes, components and all-cause death according to SGLT2i use at baseline.
Secondary endpoints related to kidney outcomes did not show significant between-group differences. Safety analyses revealed comparable rates of serious adverse events between oral semaglutide and placebo groups (47.9% vs. 50.3%) and a marginally higher rate of gastrointestinal disorders with semaglutide (5.0% vs. 4.4%), consistent with known GLP-1 RA side effects.
Together, these findings support that oral semaglutide effectively reduces cardiovascular risk independent of concomitant SGLT2i treatment, and the combination is well tolerated.
Expert Commentary
The SOUL trial’s robust design and large cohort with extended follow-up provide compelling evidence for the cardiovascular benefits of oral semaglutide in a high-risk T2DM population. Importantly, this study addresses a critical clinical question about the joint use of GLP-1 RAs and SGLT2i, demonstrating additive, safe use rather than diminishing efficacy or increased risk.
Contemporary guidelines endorse both GLP-1 RAs and SGLT2i for cardiovascular risk reduction in T2DM, yet clinicians often face uncertainty regarding simultaneous use. The SOUL data fill this gap, encouraging a more integrated approach to multimodal risk management.
Limitations include the exploratory nature of the subgroup analyses regarding SGLT2i use and the absence of power to detect small differences in renal outcomes. Ongoing studies may clarify long-term renal benefits further. Mechanistically, GLP-1 RAs reduce atherosclerosis progression, and SGLT2i improve hemodynamics and reduce heart failure risk, thus complementary pathways likely underpin combined benefits.
Conclusion
Oral semaglutide significantly reduces major adverse cardiovascular events in patients with type 2 diabetes and high cardiovascular risk, regardless of background treatment with SGLT2 inhibitors. The combination is safe and well tolerated. These findings strengthen the role of oral semaglutide as a key GLP-1 RA agent in comprehensive cardiovascular risk mitigation strategies in T2DM and support clinical guideline incorporation for combined GLP-1 RA and SGLT2i therapy where appropriate.
References
- McGuire DK, Marx N, Mulvagh SL, et al. Oral Semaglutide and Cardiovascular Outcomes in High-Risk Type 2 Diabetes. N Engl J Med. 2025;392(20):2001-2012. doi:10.1056/NEJMoa2501006 IF: 78.5 Q1
- Marx N, Deanfield JE, Mann JFE, et al. Oral Semaglutide and Cardiovascular Outcomes in People With Type 2 Diabetes, According to SGLT2i Use: Prespecified Analyses of the SOUL Randomized Trial. Circulation. 2025;151(23):1639-1650. doi:10.1161/CIRCULATIONAHA.125.074545 IF: 38.6 Q1 PDF (513.9 KB)