Highlight
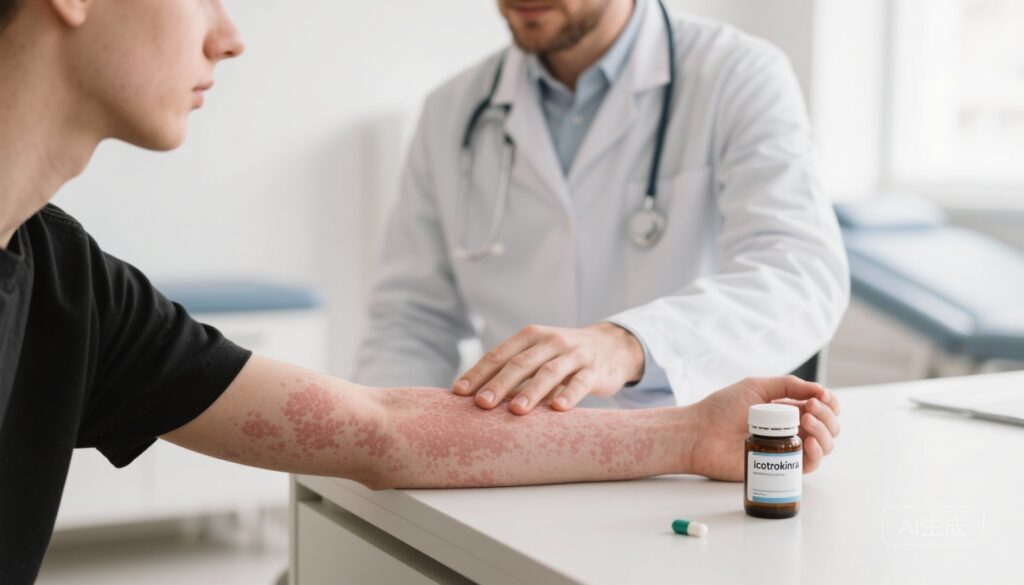
Icotrokinra, a once‑daily oral peptide that selectively antagonizes the interleukin‑23 receptor (IL‑23R), produced rapid and clinically meaningful skin clearance in adults and adolescents with moderate‑to‑severe plaque psoriasis. Coprimary endpoints were met across multiple phase‑3 trials (ICONIC and ICONIC‑ADVANCE), with IGA 0/1 rates of approximately 65–70% and PASI 90 rates of 50–57% at week 16. Short‑term safety through 16–24 weeks was comparable to placebo and favorable versus an oral TYK2 inhibitor in pooled analyses, but longer follow‑up is required to define the full benefit‑risk profile.
Background and Unmet Need
Plaque psoriasis is a chronic, immune‑mediated inflammatory skin disease that appreciably reduces quality of life and is associated with systemic comorbidities including psoriatic arthritis, cardiometabolic disease, and mental‑health burden. Over the past decade, biologic therapies targeting IL‑23 (p19), IL‑17, and TNF have transformed outcomes, enabling high rates of skin clearance. However, injectables remain the dominant format for the most efficacious agents, and there is ongoing demand for effective, orally administered therapies with favorable safety profiles and patient convenience. Icotrokinra represents a novel approach: a small, orally administered peptide designed to bind selectively to the IL‑23 receptor, thereby blocking IL‑23 signaling upstream of Th17 effectors implicated in psoriasis pathogenesis.
Study Designs and Populations
ICONIC‑LEAD (NEJM report)
The pivotal randomized, double‑blind, placebo‑controlled phase‑3 trial enrolled adults and adolescents (≥12 years) with moderate‑to‑severe plaque psoriasis defined by body‑surface area involvement ≥10%, baseline PASI ≥12, and Investigator’s Global Assessment (IGA) ≥3. Participants were randomized 2:1 to oral icotrokinra 200 mg once daily through week 24 or placebo through week 16 with subsequent transition to icotrokinra. Coprimary endpoints were the proportions achieving IGA 0/1 (clear or almost clear, with ≥2‑point improvement) and PASI 90 (≥90% reduction) at week 16.
ICONIC‑ADVANCE 1 and 2 (Lancet report)
Two global phase‑3 trials (ADVANCE 1 and 2) randomized adults with at least 26 weeks’ psoriasis history to icotrokinra 200 mg once daily, placebo, or deucravacitinib 6 mg daily (an oral TYK2 inhibitor). Randomization schemes were 2:1:2 (ADVANCE 1) and 4:1:4 (ADVANCE 2). Placebo and deucravacitinib arms transitioned to icotrokinra at prespecified weeks (16 or 24). Coprimary endpoints matched ICONIC‑LEAD: IGA 0/1 with ≥2‑grade improvement and PASI 90 at week 16. Trials enrolled broad multinational populations across many sites and were double‑blind and active‑comparator‑controlled.
Key Efficacy Findings
ICONIC‑LEAD (NEJM)
Among 684 randomized participants (456 icotrokinra, 228 placebo), icotrokinra produced markedly higher rates of skin clearance at week 16:
– IGA 0/1: 65% with icotrokinra vs. 8% with placebo (P<0.001).
– PASI 90: 50% vs. 4% (P<0.001).
– Complete clearance (IGA 0): 33% vs. 1%.
– PASI 100 (complete clearance): 27% vs. <1%.
These effect sizes indicate substantial clinical efficacy within 16 weeks in a mixed adult and adolescent cohort.
ICONIC‑ADVANCE 1 and 2 (Lancet)
Both ADVANCE trials met coprimary endpoints at week 16. Representative results:
– ADVANCE 1: IGA 0/1 in 68% (311 icotrokinra) vs. 11% (156 placebo); PASI 90 in 55% vs. 4% (both p<0.0001).
– ADVANCE 2: IGA 0/1 in 70% (322 icotrokinra) vs. 9% (82 placebo); PASI 90 in 57% vs. 1% (both p<0.0001).
Across both ADVANCE studies, icotrokinra produced consistent and reproducible efficacy with IGA 0/1 rates of roughly two‑thirds of treated patients and PASI 90 approaching 55–57% by week 16.
Comparative Context
Although direct head‑to‑head efficacy comparisons with established injectable anti‑IL‑23 p19 monoclonal antibodies are not reported in these manuscripts, the magnitude of response with icotrokinra approaches the levels commonly seen with modern biologics at similar time points. The ICONIC‑ADVANCE program also included deucravacitinib as an active oral comparator; safety data up to week 24 showed a somewhat higher adverse‑event burden with deucravacitinib than with icotrokinra (65% vs. 57% to week 24), but efficacy comparisons versus deucravacitinib were not detailed in the provided summary and should be interpreted when full comparative analyses are available.
Safety and Tolerability
Across trials, the overall incidence of adverse events through week 16 was similar between icotrokinra and placebo. In ICONIC‑LEAD, 49% of participants in each group reported at least one adverse event to week 16. The most commonly reported events were nasopharyngitis and upper respiratory tract infection. Exposure‑adjusted incidence remained consistent through week 24.
In the pooled ADVANCE analyses, adverse events to week 16 occurred in 48% of icotrokinra recipients and 57% of placebo recipients; the most common events were nasopharyngitis and upper respiratory tract infection. By week 24, fewer adverse events were recorded with icotrokinra (57%) than with deucravacitinib (65%) in the pooled dataset.
No unexpected safety signals were reported in these short‑term analyses; however, key safety considerations for any therapy that modulates IL‑23 signaling include infection risk (particularly opportunistic infections), potential effects on tumor surveillance, and immunogenicity. Longitudinal data (months to years) and post‑marketing surveillance will be necessary to characterize rare but serious events, effects on comorbid conditions, and sustainability of response.
Expert Commentary and Interpretation
The ICONIC program demonstrates that oral, receptor‑targeted blockade of IL‑23 can achieve clinically meaningful and rapid skin clearance in a large, diverse psoriasis population, including adolescents. Strengths of these programs include randomized, double‑blind design; large sample sizes; consistent coprimary endpoints used across trials; and inclusion of an active oral comparator in the ADVANCE studies.
Mechanistically, IL‑23 is a central cytokine in the Th17 pathway and a validated therapeutic target in psoriasis. Unlike monoclonal antibodies against the IL‑23 p19 subunit, icotrokinra binds the IL‑23 receptor, a different node in the same pathway. An oral peptide format could improve patient convenience and uptake, especially for individuals averse to injections.
Key interpretative caveats include the relatively short primary endpoint (week 16) for long‑term chronic disease management, placebo crossover designs that limit long‑term placebo comparisons, and limited disclosure in the summaries regarding efficacy versus the active comparator deucravacitinib. Additionally, safety evaluation beyond 24 weeks is required to assess risks including serious infections, malignancy, laboratory abnormalities, and rare immune complications.
Clinical and Research Implications
For clinicians, icotrokinra may soon provide an oral option with efficacy approximating that of modern biologics for many patients with moderate‑to‑severe plaque psoriasis. Its role relative to established injectables will depend on long‑term efficacy durability, safety, patient preference, cost and access, and comparative effectiveness trials. Important unanswered questions include:
– Durability of PASI 90/PASI 100 responses beyond 6–12 months.
– Long‑term safety, including serious infection, malignancy, and cardiovascular events.
– Performance in subpopulations (e.g., those with psoriatic arthritis, obesity, prior biologic failures, latent tuberculosis).
– Comparative effectiveness and cost‑effectiveness versus injectable IL‑23 p19 inhibitors and oral TYK2 inhibitors.
Further phase‑3 extension data and real‑world evidence will be critical for answering these questions.
Limitations of the Current Evidence
The reported data are robust for the 16‑week primary endpoint, but the primary limitations are the short follow‑up for chronic disease outcomes and relatively limited public detail on certain subgroup analyses and comparisons with active oral therapy. Trial populations often exclude patients with significant comorbidities or recent serious infections, which can limit generalizability. Finally, while pooled safety comparisons to 24 weeks are reassuring, long‑term surveillance is essential for assessing rare adverse events.
Conclusions
Icotrokinra is an innovative oral IL‑23 receptor antagonist that produced rapid and substantial skin clearance in phase‑3 trials of adults and adolescents with moderate‑to‑severe plaque psoriasis. Coprimary endpoints were consistently met across large multinational trials, and short‑term safety was broadly comparable to placebo and favorable in pooled comparisons with an oral TYK2 inhibitor. These data support icotrokinra as a promising oral therapeutic option; however, clinicians should await longer‑term efficacy and safety data, head‑to‑head comparative analyses, and real‑world experience to fully define its place in the treatment algorithm.
Funding and ClinicalTrials.gov
The ICONIC and ICONIC‑ADVANCE programs were funded by Johnson & Johnson. Clinical trial registrations include NCT06095115 (ICONIC‑LEAD), NCT06143878 (ICONIC‑ADVANCE 1), and NCT06220604 (ICONIC‑ADVANCE 2).
References
Bissonnette R, Soung J, Hebert AA, et al. Oral Icotrokinra for Plaque Psoriasis in Adults and Adolescents. N Engl J Med. 2025 Nov 6;393(18):1784-1795. doi: 10.1056/NEJMoa2504187 IF: 78.5 Q1 . PMID: 41191940 IF: 78.5 Q1 .
Gold LS, Armstrong AW, Bissonnette R, et al. Once-daily oral icotrokinra versus placebo and once-daily oral deucravacitinib in participants with moderate-to-severe plaque psoriasis (ICONIC-ADVANCE 1 & 2): two phase 3, randomised, placebo-controlled and active-comparator-controlled trials. Lancet. 2025 Sep 27;406(10510):1363-1374. doi: 10.1016/S0140-6736(25)01576-4 IF: 88.5 Q1 . PMID: 40976249 IF: 88.5 Q1 .