Introduction: The Shifting Landscape of Breast Cancer Care
For decades, the journey of a patient with hormone receptor-positive (HR+), human epidermal growth factor receptor 2-negative (HER2-) metastatic breast cancer followed a predictable, if challenging, path. Endocrine therapy, often combined with CDK4/6 inhibitors, served as the frontline defense. However, when the cancer eventually evolved to resist these treatments, the options grew narrower, often leading to traditional chemotherapy—a modality known as much for its systemic toxicity as for its therapeutic efficacy. Today, we are witnessing a paradigm shift. The rise of antibody-drug conjugates (ADCs) is redefining what it means to live with advanced breast cancer. At the heart of this transformation is the TROPION-Breast01 study, which recently released its final overall survival analysis, providing a comprehensive look at the role of datopotamab deruxtecan (Dato-DXd) in this patient population.
The Case of Sarah: Navigating Resistance
To understand the clinical significance of these findings, consider the story of Sarah, a 54-year-old teacher from Ohio. Diagnosed with HR+/HER2- metastatic breast cancer four years ago, Sarah had successfully managed her disease with letrozole and palbociclib for nearly three years. When her scans eventually showed new lesions in her liver, she transitioned to fulvestrant and alpelisib. However, after eight months, her disease progressed again. Sarah’s oncologist explained that the next step would likely be chemotherapy—eribulin or capecitabine. For Sarah, the prospect of chemotherapy brought significant anxiety about hair loss, severe fatigue, and the risk of infection. Like many patients in her position, Sarah represents the ‘previously treated’ population for whom TROPION-Breast01 sought to find a better alternative.
Understanding Datopotamab Deruxtecan: The Trojan Horse Mechanism
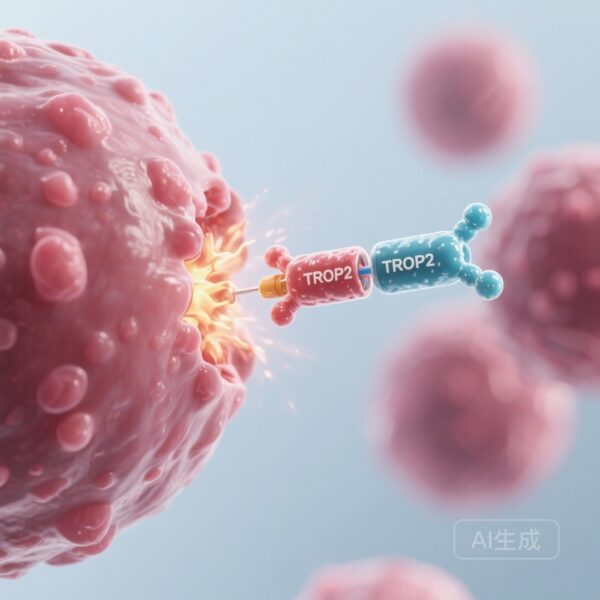
Datopotamab deruxtecan belongs to a sophisticated class of drugs called antibody-drug conjugates. Often described as ‘biological missiles’ or ‘Trojan horses,’ ADCs consist of three parts: a monoclonal antibody that targets a specific protein on cancer cells, a cytotoxic ‘payload’ (chemotherapy drug), and a linker that joins them. Dato-DXd specifically targets TROP2 (trophoblast cell surface antigen 2), a protein that is overexpressed in the vast majority of HR+ breast cancers. Once the antibody binds to TROP2 on the cancer cell surface, the entire complex is internalized. Inside the cell, the linker is cleaved, releasing a potent topoisomerase I inhibitor directly into the tumor. This targeted approach allows for a higher concentration of the drug within the cancer cells while sparing healthy tissue, theoretically reducing the systemic side effects seen with conventional ‘free’ chemotherapy.
The TROPION-Breast01 Study: Design and Execution
TROPION-Breast01 was a global, phase 3, open-label, randomized trial designed to compare Dato-DXd with the investigator’s choice of chemotherapy (ICC) in patients like Sarah. The study enrolled 732 patients who had progressed on endocrine therapy and had received one to two prior lines of chemotherapy for metastatic disease. The primary endpoints were dual: progression-free survival (PFS)—the length of time during and after treatment that a patient lives with the disease but it does not get worse—and overall survival (OS). The chemotherapy choices in the control arm included common agents like eribulin, capecitabine, vinorelbine, or gemcitabine.
Key Findings: A Triumph in Progression-Free Survival
The primary results of TROPION-Breast01 were highly encouraging. Dato-DXd demonstrated a statistically significant and clinically meaningful improvement in PFS compared to chemotherapy. The risk of disease progression or death was reduced by 37% (Hazard Ratio [HR] 0.63). For patients, this meant a median PFS of 6.9 months with Dato-DXd compared to 4.9 months with standard chemotherapy. While a two-month difference might seem modest to a layperson, in the context of advanced, pre-treated metastatic disease, it represents a substantial delay in the progression of a life-threatening illness. Furthermore, the objective response rate (ORR) was significantly higher in the Dato-DXd group (36.4%) compared to the chemotherapy group (22.9%), indicating that more patients saw their tumors shrink significantly.
The Overall Survival Paradox: Why the Numbers Tell a Complex Story
The final analysis of the study focused on overall survival (OS). The results showed that median OS was similar between the two groups (HR 1.01), meaning the trial did not reach statistical significance for this specific endpoint. At first glance, this might seem like a setback. However, medical researchers emphasize the importance of context. In the years since the trial began, several other ADCs—such as trastuzumab deruxtecan and sacituzumab govitecan—have been approved and integrated into standard care. The data revealed a significant ‘imbalance’ in subsequent treatments: 24% of the patients in the chemotherapy arm went on to receive another ADC after the study, compared to only 12.3% in the Dato-DXd arm. This ‘cross-over’ effect often makes it difficult to show a survival advantage for the initial drug because the control group is effectively receiving similar targeted therapies later in their journey. Despite the OS results, other secondary efficacy markers, such as time to first and second subsequent therapies, continued to favor Dato-DXd.
Safety and Quality of Life: A Balanced Profile
For patients like Sarah, the ‘how’ of a treatment is often as important as the ‘how long.’ The safety profile of Dato-DXd in TROPION-Breast01 was a major highlight. Grade 3 or higher treatment-related adverse events (TRAEs) occurred in only 20.8% of patients receiving Dato-DXd, compared to 44.7% of those receiving chemotherapy. This suggests that Dato-DXd is generally better tolerated than standard cytotoxic agents. However, the side effects are different. While chemotherapy patients struggled more with neutropenia (low white blood cell counts) and hair loss, Dato-DXd patients experienced more nausea (51.1%) and stomatitis (mouth sores, 50%). Most of these were low-grade, but they require proactive management.
| Adverse Event (Any Grade) | Dato-DXd (%) | ICC (%) |
|---|---|---|
| Nausea | 51.1 | 23.6 |
| Stomatitis (Mouth Sores) | 50.0 | 12.8 |
| Neutropenia (Grade ≥3) | 0.8 | 30.8 |
| Alopecia (Hair Loss) | 36.4 | 20.5 |
Expert Insights: Where Do We Go From Here?
Dr. Aditya Bardia, a lead investigator of the study, noted that the totality of the data supports Dato-DXd as a viable new treatment option. The significant PFS benefit and the manageable safety profile address a high unmet need for patients who have exhausted endocrine-based therapies. The challenge for clinicians moving forward will be ‘sequencing’—deciding which ADC to use first and how to manage the cumulative toxicities of these powerful new agents. As more drugs target TROP2 and other markers, the goal is to create a chronic disease management model for metastatic breast cancer, where patients can rotate through targeted therapies for years while maintaining a high quality of life.
Conclusion: A Step Toward Personalized Medicine
The TROPION-Breast01 study represents a pivotal moment in the treatment of HR+/HER2- breast cancer. While the overall survival data remind us of the complexities of clinical research in an era of rapid innovation, the progression-free survival and safety results are a win for patients. For Sarah and many others like her, the emergence of datopotamab deruxtecan means one more effective tool in the kit, one more opportunity to delay the hardships of traditional chemotherapy, and one more reason to hold onto hope. As oncology moves further toward precision medicine, the focus remains clear: delivering the right drug to the right target at the right time.
References
1. Pistilli B, Jhaveri K, Im SA, et al. Datopotamab deruxtecan versus chemotherapy in previously treated inoperable/metastatic hormone-receptor-positive HER2-negative breast cancer: final overall survival analysis of the phase 3 TROPION-Breast01 study. Ann Oncol. 2025;S0923-7534(25)06337-9.
2. Bardia A, Jhaveri K, Im SA, et al. Datopotamab Deruxtecan Versus Chemotherapy in Previously Treated Inoperable/Metastatic Hormone Receptor-Positive Human Epidermal Growth Factor Receptor 2-Negative Breast Cancer: Primary Results From TROPION-Breast01. J Clin Oncol. 2025;43(3):285-296.
3. Hunter FW, Barker HR, Lipert B, et al. Mechanisms of resistance to antibody-drug conjugates. Nat Rev Clin Oncol. 2020;17(4):239-255.