Highlights
• Respiratory viral infections (influenza and SARS‑CoV‑2) can awaken dormant disseminated breast cancer cells (DCCs) in lung parenchyma, promoting proliferation and long-term metastatic outgrowth in mouse models.
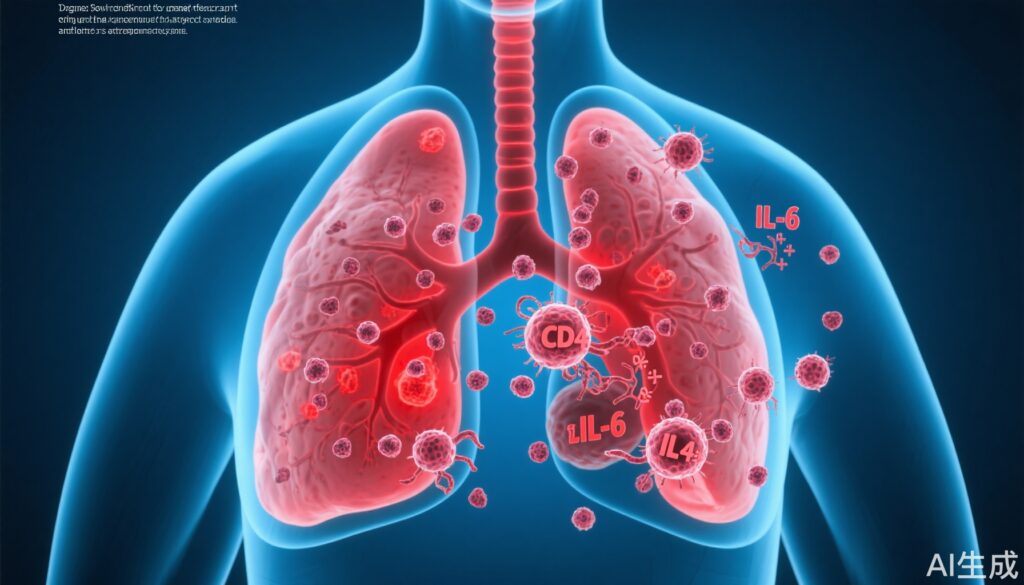
• IL‑6 signaling is necessary for the early reactivation step; CD4+ T cells are required to sustain the outgrowth by suppressing anti‑tumor CD8+ responses.
• Human registry analyses (including UK Biobank and Flatiron Health data queried by the study authors) showed higher cancer‑related mortality and increased risk of lung metastasis among cancer patients who had documented SARS‑CoV‑2 infection.
Study background and disease burden
Breast cancer is one of the most common malignancies worldwide and, even after apparent remission, disseminated cancer cells (DCCs) can persist in distant organs such as the lung, bone, and liver for years to decades in a non‑proliferative “dormant” state. Reactivation of these DCCs is a principal cause of late metastatic relapse and cancer mortality. Understanding triggers that break dormancy could inform strategies to prevent recurrence.
During the early COVID‑19 pandemic, observational signals emerged of increased cancer‑related excess deaths that could not be fully explained by direct SARS‑CoV‑2 mortality or delays in diagnosis/treatment. This raised the hypothesis that pulmonary infections — common causes of localized inflammation — might directly alter the lung microenvironment and promote awakening of dormant DCCs, thereby increasing metastatic relapse. The Nature paper by the University of Colorado group (Respiratory viral infections awaken metastatic breast cancer cells in lungs, Nature, 2025) directly tests that hypothesis in preclinical models and complements it with human registry analyses.
Study design
This translational study combined mechanistic mouse experiments, in vitro assays, and retrospective analysis of human clinical databases.
Preclinical models:
• Mouse models: Two independent metastatic breast cancer mouse models known to seed dormant DCCs in the lung were used. Tumor cells remained largely non‑proliferative (dormant) in lungs prior to viral challenge.
• Viral challenge: Experimental infection with influenza virus and, in parallel experiments, with SARS‑CoV‑2 in appropriate mouse models.
• Interventions and manipulations: Genetic ablation of tumor cell IL‑6 production, in vitro IL‑6 treatments, and CD4+ T‑cell depletion were employed to dissect mechanisms. Immune phenotyping and gene expression analyses were used to evaluate immune cell dynamics (CD4+ and CD8+ T cells) and cytokine signaling.
Human data analysis:
• Databases: The authors queried the UK Biobank (covering multiple cancer types) and the Flatiron Health electronic‑health‑record–derived database (breast cancer cohort) to evaluate associations between documented SARS‑CoV‑2 infection and subsequent cancer‑related outcomes.
• Outcomes: Cancer‑related mortality and the incidence of new pulmonary metastases were assessed and compared between infected and non‑infected cancer patients, with adjustment for available covariates.
Key endpoints included changes in number and proliferation status of DCCs in lungs post‑infection (mouse), tumor growth in vitro with IL‑6 exposure, immune cell composition changes, and hazard/odds estimates for human outcomes.
Key findings
Preclinical results (mouse models):
• Rapid DCC reactivation after respiratory viral infection: In both influenza and SARS‑CoV‑2 mouse challenge experiments, previously dormant DCCs in the lung displayed increased proliferation as early as 15 days after infection. Proliferation persisted—measurable for months in the experimental follow‑up—culminating in overt metastatic lesions.
• IL‑6 dependence: DCCs and lung microenvironments showed activation of IL‑6–dependent signaling pathways following viral infection. Tumor cells genetically engineered to lack IL‑6 production showed significantly fewer proliferating DCCs after viral challenge compared with IL‑6–competent controls. In vitro, exogenous IL‑6 increased breast cancer cell growth, supporting a direct pro‑proliferative effect.
• Temporal dynamics of IL‑6 and sustained outgrowth: Although lung IL‑6 protein levels were markedly elevated early after infection, IL‑6 levels normalized within ~15 days in lungs in the reported experiments; nevertheless, DCC proliferation persisted for much longer, indicating IL‑6 may initiate reactivation but additional processes sustain metastatic outgrowth.
• Role of CD4+ T cells in maintenance: After viral infection, CD4+ T cells were recruited to lung regions containing DCCs. Experimental depletion of CD4+ T cells did not change the early (day 9) increase in proliferating DCCs but did reduce the number of proliferating DCCs at later timepoints (day 28), indicating CD4+ T cells are required to maintain reactivated growth but are not the trigger that initiates reactivation.
• CD8+ T‑cell suppression by CD4+ cells: Gene expression and immune phenotyping showed that CD4+ T‑cell depletion led to an increase in CD8+ T cells in the lung. The authors infer that CD4+ T cells recruited during infection may restrain CD8+‑mediated anti‑tumor surveillance, allowing sustained DCC expansion.
Human registry findings:
• Concordant clinical signal: Using UK Biobank and Flatiron data, the study reported that cancer patients with documented SARS‑CoV‑2 infection had significantly higher cancer‑related mortality and an increased risk of developing lung metastases compared with non‑infected cancer patients. The authors emphasize that these observational associations are consistent with the preclinical mechanism but cannot in isolation prove causation given potential confounding.
Safety and adverse‑effect observations:
• The preclinical paper focused on mechanism and did not report classic drug or treatment safety data. From a translational standpoint, the study raises potential therapeutic hypotheses (e.g., targeting IL‑6 signaling or modulating CD4+/CD8+ balance), but such strategies would require careful evaluation because of the central role of these pathways in viral defense and tissue repair.
Expert commentary and mechanistic plausibility
Biological plausibility: The findings align with well‑established links between inflammation and cancer progression. Cytokines such as IL‑6 are central mediators of acute inflammatory responses and have long been implicated in tumor cell proliferation, survival, and modulation of the tumor microenvironment (reviewed in Grivennikov et al., Cell 2010). IL‑6 can act directly on tumor cells through STAT3 activation to promote proliferation and survival, and indirectly by shaping immune cell recruitment and stromal remodeling.
Immune modulation: The observation that CD4+ T cells support sustained DCC outgrowth by limiting CD8+‑mediated control is consistent with the nuanced roles of CD4+ subsets—helper and regulatory—during inflammation. CD4+ regulatory T cells (Tregs) can suppress cytotoxic responses and promote tissue repair; in the context of a post‑viral inflammatory milieu, such suppression could inadvertently shield reactivated tumor cells from elimination.
Clinical implications: If confirmed and generalized, these data suggest respiratory infections are a modifiable risk factor for metastatic relapse, particularly for cancers that commonly seed the lung. This raises practical questions for survivorship care and infection prevention strategies in cancer patients and survivors.
Limitations and alternative interpretations:
• Preclinical limitations: Mouse models do not fully reproduce human cancer biology, immune repertoire, prior therapies, comorbidities, or the complexity of human viral exposures. The timing, dose, and strains of viruses used in experiments influence immune responses and may not match typical human infections.
• Human data constraints: Observational registry analyses are subject to confounding (e.g., differences in health status, socioeconomic factors, cancer stage, treatment interruptions, vaccination status). Reverse causation (e.g., people with more fragile health being both more likely to get severe COVID‑19 and to die of cancer) remains a possibility despite adjustment.
• Therapeutic caution: IL‑6 blockade (e.g., tocilizumab) was used in COVID‑19 to treat hyperinflammation; however, translating IL‑6 inhibitors to reduce metastatic risk would be complex, as IL‑6 is important for infection control and tissue repair. Similarly, broadly depleting CD4+ T cells would be immunosuppressive and unsafe.
Context with prior literature: Prior studies documented increases in excess cancer deaths during the pandemic and reductions in screening/diagnosis (e.g., Kaufman et al., JAMA Netw Open 2020; Maringe et al., Lancet Oncol 2020). The Nature study adds a mechanistic layer suggesting infections themselves, independently of diagnostic and treatment disruptions, could contribute biologically to worse cancer outcomes.
Clinical and research implications
For clinicians:
• Preventive emphasis: Reinforce respiratory infection prevention in cancer survivors—vaccination (influenza, COVID‑19 per guidelines), early antiviral therapy when indicated, and standard infection control measures—particularly in patients with cancers known to seed the lung.
• Vigilance during and after respiratory infections: Consider closer surveillance for signs or symptoms of metastatic relapse in appropriate patients after a serious respiratory infection, balanced against the risks and costs of over‑testing.
• Therapeutic research caution: Existing immunomodulatory drugs (IL‑6 inhibitors, immune‑checkpoint agents) suggest potential translational paths but should not be repurposed empirically for metastasis prevention without rigorous clinical trials.
For researchers:
• Reproduce and extend: Independent replication in additional tumor types and in models capturing prior therapies and immune aging is essential.
• Mechanistic dissection: Identify the sustaining factors beyond transient IL‑6 elevation (matrix remodeling, stromal cell changes, lasting T‑cell polarization) that permit prolonged outgrowth.
• Interventional trials: Carefully designed translational trials could test peri‑infection strategies (for example, targeted cytokine modulation timed to infection) but must weigh infection control and oncologic safety.
Conclusion
The Nature paper presents compelling preclinical evidence that common respiratory viruses can wake dormant breast cancer DCCs in the lung and trigger durable metastatic growth, mediated initially by IL‑6 and later sustained by CD4+ T‑cell–dependent suppression of CD8+ control. Observational human data presented alongside the mouse studies are consistent with an increased cancer‑related mortality and pulmonary metastasis risk following SARS‑CoV‑2 infection. While causality in humans remains to be fully established, the work identifies a potentially modifiable pathway linking infection, inflammation, and metastatic relapse and underscores the importance of infection prevention and targeted mechanistic research in cancer survivorship.
References
1. University of Colorado authors. Respiratory viral infections awaken metastatic breast cancer cells in lungs. Nature. 2025. https://www.nature.com/articles/s41586-025-09332-0
2. Kaufman HW, Chen Z, Niles J, Fesko Y. Changes in the Number of US Patients With Newly Identified Cancer Before and During the Coronavirus Disease 2019 (COVID‑19) Pandemic. JAMA Netw Open. 2020;3(8):e2017267. doi:10.1001/jamanetworkopen.2020.172673. Maringe C, Spicer J, Morris M, et al. The impact of the COVID‑19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population‑based, modelling study. Lancet Oncol. 2020;21(8):1023‑1034. doi:10.1016/S1470-2045(20)30388-04. Grivennikov SI, Greten FR, Karin M. Immunity, Inflammation, and Cancer. Cell. 2010;140(6):883‑899. doi:10.1016/j.cell.2010.01.0255. Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331(6024):1565‑1570. doi:10.1126/science.1203486