Highlights
- Large prospective data show that incident myocardial infarction (MI) doubles the risk of developing late-onset epilepsy (LOE) in stroke-free older adults.
- LOE similarly signals elevated risk for subsequent MI and nonstroke vascular death, indicating bidirectional associations suggestive of systemic vascular pathology.
- Findings support considering LOE as a vascular risk equivalent, highlighting the need for intensified vascular risk factor control and interdisciplinary clinical attention.
- Further research is needed to validate these findings in diverse populations and elucidate underlying pathophysiological mechanisms.
Background
Late-onset epilepsy (LOE), defined as epilepsy with onset after middle age, is increasingly recognized as a consequential neurological condition in aging populations. Epidemiological studies have established cerebrovascular disease as a prominent etiological factor for LOE, as vascular injury or ischemia alters excitability thresholds and neuronal network function, predisposing to seizures. Notably, systemic vascular disease often involves multiple vascular beds, not limited to cerebral vasculature, raising the question of whether LOE is a marker reflecting broader systemic vascular risk rather than isolated brain pathology.
Myocardial infarction (MI) is a leading cause of morbidity and mortality globally and represents systemic atherosclerotic and thrombotic disease that shares common risk factors with cerebrovascular disorders such as hypertension, diabetes, and dyslipidemia. The interplay between MI and LOE remains poorly characterized, particularly in populations without prior stroke—a major confounder of post-stroke epilepsy. Understanding potential bidirectional associations has implications for risk stratification, prevention, and clinical management strategies.
Key Content
Prospective Evidence From the Northern Manhattan Study (NOMAS)
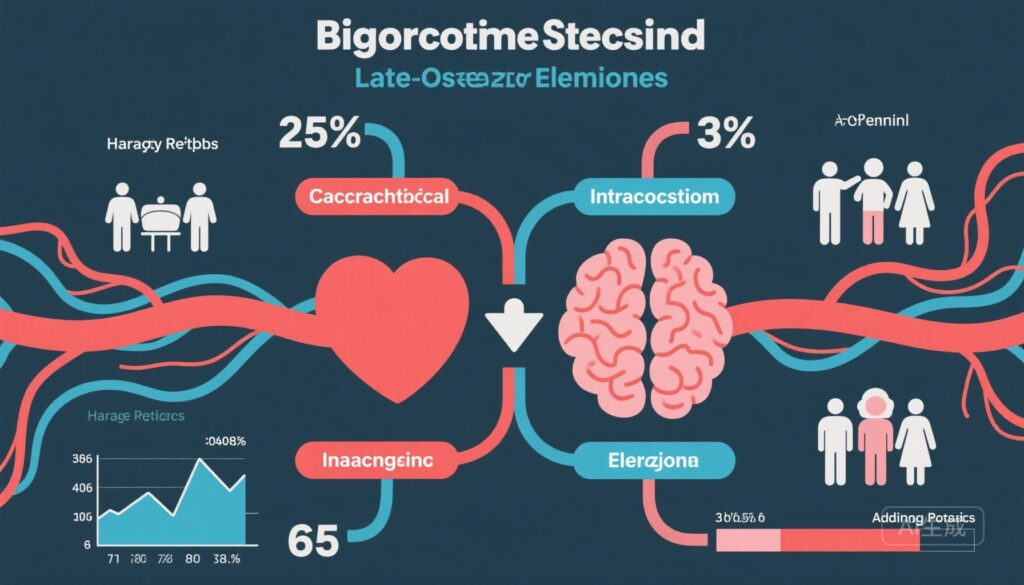
The study by Thacker et al. (2025) analyzed 3,174 stroke-free community-dwelling adults aged 40 years and older enrolled between 1993 and 2008 in NOMAS, with up to 30 years of follow-up (mean 14 years). Baseline exclusion criteria included prior history of stroke, MI, or epilepsy to isolate incident cases. Over the study period, 296 (9.3%) developed incident MI, 120 (3.8%) developed incident LOE, and 794 (25.0%) experienced nonstroke vascular death.
Using Cox proportional hazards models with censoring for incident stroke, the investigators observed:
- Incident MI was associated with a more than twofold increased risk of subsequent LOE (adjusted hazard ratio [aHR] 2.12, 95% CI 1.06–4.25; p=0.035).
- Conversely, incident LOE trended toward nearly double risk of subsequent MI (aHR 1.99, 95% CI 0.98–4.05; p=0.059), approaching but not achieving conventional statistical significance.
- Incident LOE was significantly associated with increased nonstroke vascular mortality (aHR 2.82, 95% CI 2.09–3.80; p<0.001), indicating a heightened systemic vascular burden beyond stroke-related causes.
Sensitivity analysis reinforced robustness of these findings. Importantly, the analyses adjusted rigorously for confounders including demographics, vascular risk factors, and health behaviors.
Contextualizing with Related Literature and Expert Commentary
Prior studies have established stroke as a major cause of LOE, with mechanisms involving cortical ischemia, gliosis, and network hyperexcitability. While MI has not previously been emphasized as a direct risk factor for LOE absent stroke, this study implicates systemic vascular pathophysiology, including microvascular cerebral ischemia and endothelial dysfunction, as contributory. Stefanidou and Friedman (2025) editorialize that LOE may serve as a sentinel event reflecting multisystem vascular disease paralleling MI and ischemic heart disease.
These insights align with pathobiological concepts of shared atherosclerotic and thrombotic mechanisms impacting both cerebral and cardiac circulation. Vascular endothelial injury, inflammatory cascades, and impaired cerebral autoregulation in patients with MI could predispose to epileptogenic substrates even in the absence of overt stroke. Conversely, LOE itself may reflect a state of heightened vascular vulnerability, explaining the trend toward increased incident MI and the strong association with nonstroke vascular death.
Implications for Clinical Practice and Vascular Risk Management
The bidirectional associations prompt reconsideration of LOE as a clinically relevant vascular risk equivalent akin to stroke in cardiovascular risk assessment tools. For clinicians, a new diagnosis of LOE in an older adult should trigger comprehensive evaluation and aggressive management of vascular risk factors, including hypertension, hyperlipidemia, diabetes, and lifestyle modifications—potentially reducing subsequent cardiovascular morbidity and mortality.
Moreover, the findings suggest that patients post-MI warrant neurological surveillance for possible development of LOE, especially as improved cardiac care increases survival and highlights late neurological sequelae.
Research Domain and Methodological Considerations
The strength of the NOMAS study lies in its prospective design, lengthy follow-up, comprehensive phenotyping, and adjustment for incident stroke censoring, which minimizes confounding by stroke-related epilepsy. Limitations include modest LOE event numbers limiting statistical power, absence of detailed imaging or biomarker corroboration of cerebral microvascular disease, and generalizability concerns given cohort demographic and geographic specificity.
Additional studies across diverse populations employing neuroimaging, electrophysiological characterization, and mechanistic biomarker studies are warranted to elucidate causality, temporal progression, and potential therapeutic targets.
Expert Commentary
This study marks a significant step toward recognizing LOE not merely as a neurological disorder isolated to brain parenchyma but as a systemic vascular disease manifestation. The emerging concept that MI and LOE are intertwined reflects contemporary understanding of aging-related multisystem vascular pathologies.
Clinicians should be aware that LOE diagnosis warrants vigilance beyond seizure control to include cardiovascular risk stratification and preventive strategies. The observed near-significance of MI risk after LOE warrants larger confirmatory studies.
Mechanistically, microvascular ischemia, blood-brain barrier dysfunction, inflammation, and endothelial impairment could bridge cardiac and cerebral vascular pathologies, serving as fertile grounds for translational research.
Guidelines may in the future incorporate LOE into vascular risk assessment and management algorithms, potentially improving holistic care of older adults at the nexus of neurology and cardiology.
Conclusion
The bidirectional relationships between incident myocardial infarction and late-onset epilepsy demonstrated in the Northern Manhattan Study underscore LOE as a potential marker and consequence of systemic vascular disease. Recognizing LOE as a vascular risk equivalent expands the conceptualization of epilepsy in older adults and offers new avenues for integrated vascular risk reduction strategies.
Ongoing research is essential to validate these associations in diverse cohorts, characterize underlying biological mechanisms, and translate findings into clinical practice guidelines. Ultimately, this integrated vascular-neurological approach promises to optimize outcomes in aging populations vulnerable to both cardiac and cerebral vascular diseases.
References
- Thacker EL, Choi H, Strobino K, Liu M, Misiewicz S, Beard JD, Di Tullio MR, Rundek T, Elkind MSV, Gutierrez J. Associations of Late-Onset Epilepsy With Myocardial Infarction and Nonstroke Vascular Death. Neurology. 2025 Dec 9;105(11):e214292. doi: 10.1212/WNL.0000000000214292. PMID: 41191854; PMCID: PMC12590492.
- Stefanidou M, Friedman D. Seizing the Heart: Late-Onset Epilepsy and Cardiovascular Disease in Older Adults. Neurology. 2025 Dec 9;105(11):e214391. doi: 10.1212/WNL.0000000000214391. PMID: 41191855.