Highlights
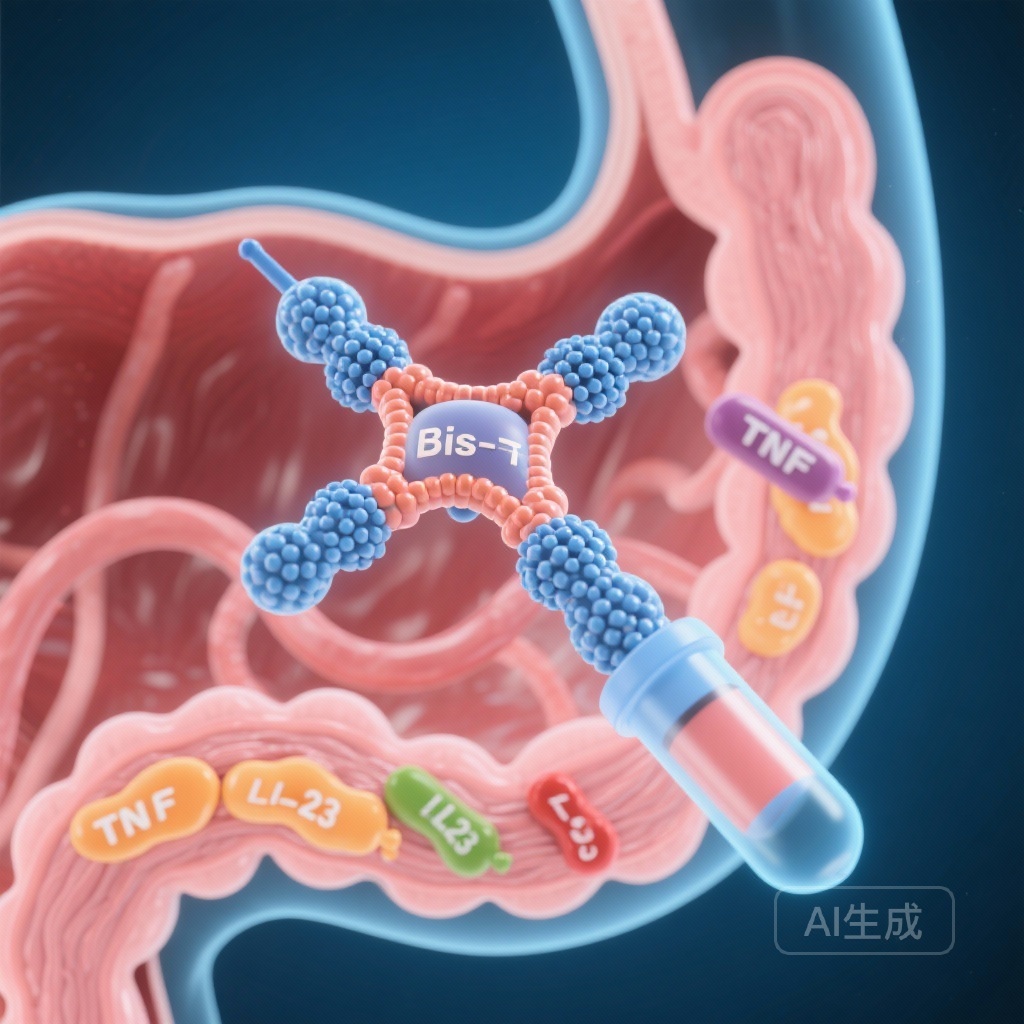
- SOR102 is a novel oral bispecific antibody targeting both Tumor Necrosis Factor (TNF) and Interleukin-23 (IL-23) p19.
- The Phase 1 trial demonstrated that SOR102 is well-tolerated in both healthy volunteers and patients with mildly-to-severely active ulcerative colitis.
- Preliminary efficacy data in Part 3 showed a 56% clinical response rate in the twice-daily SOR102 group compared to 17% in the placebo group by day 42.
- Oral delivery of SOR102 offers the potential for high local gut concentrations with minimal systemic exposure, potentially reducing systemic side effects common in parenteral biologics.
The Challenge of Refractory Ulcerative Colitis
Ulcerative colitis (UC) remains a significant therapeutic challenge for clinicians. Despite the proliferation of biologics and small molecules over the last two decades, a substantial proportion of patients—often cited as high as 40% to 60%—fail to achieve or maintain long-term remission with current standard-of-care therapies. The “therapeutic ceiling” in inflammatory bowel disease (IBD) has led researchers to explore dual-pathway inhibition and novel delivery mechanisms. Traditionally, anti-TNF agents (like infliximab) and anti-IL-23 agents (like risankizumab) have been mainstay treatments, but their parenteral administration (intravenous or subcutaneous) is associated with systemic side effects and the development of anti-drug antibodies.
Mechanistic Rationale: Why Dual Inhibition and Oral Delivery?
SOR102 represents a paradigm shift in IBD pharmacology. It is an oral bispecific antibody composed of single-domain antibodies (nanobodies) that simultaneously target TNF and the p19 subunit of IL-23. TNF is a well-established driver of acute inflammation and mucosal damage, while IL-23 plays a critical role in the Th17-mediated inflammatory cascade that sustains chronic disease. By inhibiting both pathways, SOR102 aims for a synergistic effect that may overcome the limitations of monotherapy.
Furthermore, the oral formulation of SOR102 is designed to deliver the antibody directly to the site of inflammation: the intestinal mucosa. This approach seeks to maximize local drug concentration while minimizing systemic bioavailability. In theory, this reduces the risk of systemic immunosuppression, such as serious infections or malignancies, which are perennial concerns for patients on systemic biologics.
Study Design: A Comprehensive Phase 1 Evaluation
The study was a first-in-human, double-blind, randomized, placebo-controlled trial conducted in three distinct parts across centers in the UK, Ukraine, and Georgia. The primary objective was to evaluate the safety and tolerability of SOR102.
Part 1: Single Ascending Dose (SAD)
Healthy participants were randomly assigned (6:2) to receive single doses of SOR102 (ranging from 135 mg to 3645 mg) or placebo. This phase focused on initial safety and acute pharmacokinetics.
Part 2: Multiple Ascending Dose (MAD)
Healthy participants were assigned (8:2) to receive 1215 mg of SOR102 or placebo twice daily for 7 days to assess the cumulative safety of repeated dosing.
Part 3: Patient Cohort
Patients with mildly-to-severely active UC were randomized (1:1:1) to receive SOR102 810 mg once daily, 810 mg twice daily, or placebo for a duration of 42 days. This part of the trial was crucial for observing the biological activity of the drug in an inflamed gut environment.
Key Findings: Safety and Tolerability
The trial enrolled a total of 64 participants. Across all parts of the study, SOR102 exhibited a favorable safety profile. In Part 1, treatment-emergent adverse events (TEAEs) occurred in only 13% of the SOR102 group compared to 25% of the placebo group. Most events were mild, such as flatulence or headache. Notably, only one case of mild diarrhea was deemed related to the treatment.
In the patient cohort (Part 3), TEAEs were reported in 44% of patients receiving SOR102 and 50% of those receiving placebo. While two patients in the twice-daily SOR102 group experienced a worsening of their ulcerative colitis (one of which was categorized as a serious TEAE), these were determined by investigators to be related to the underlying disease progression rather than the drug itself. Importantly, there were no clinically significant changes in vital signs, laboratory parameters, or electrocardiograms, and no deaths were reported.
Preliminary Clinical Activity: Signs of Efficacy
While Phase 1 trials are primarily designed for safety, the results from Part 3 provided compelling early evidence of clinical benefit. By day 42, the following outcomes were observed:
- Mayo Score Clinical Response: 56% of patients in the SOR102 twice-daily group achieved a response, compared to 43% in the once-daily group and 17% in the placebo group.
- Modified Mayo Score Clinical Response: 67% in the twice-daily group vs. 33% in the placebo group.
- Symptomatic Remission: Strikingly, 56% of the twice-daily group and 43% of the once-daily group achieved symptomatic remission, whereas 0% of the placebo group reached this endpoint.
These data suggest a dose-dependent relationship and indicate that SOR102 can effectively modulate the inflammatory environment of the colon when delivered orally.
Expert Commentary: A New Era for IBD Biologics?
The results of this trial are highly encouraging for the IBD community. The ability to deliver a bispecific antibody orally addresses several unmet needs. First, it offers a level of convenience that could significantly improve patient adherence compared to self-injectables or clinic-based infusions. Second, the localized delivery mechanism potentially expands the therapeutic window, allowing for potent dual inhibition without the systemic safety baggage.
However, several questions remain. As a Phase 1 trial, the sample size in the patient cohort was small, necessitating larger Phase 2 and 3 trials to confirm these efficacy signals. Furthermore, the durability of the response beyond 42 days and the long-term impact on mucosal healing need to be established. Clinicians will also be interested in how SOR102 performs in “bio-exposed” patients—those who have already failed one or more systemic biologics.
Conclusion
The Phase 1 trial of SOR102 successfully met its primary safety objectives and provided provocative early evidence of clinical efficacy in ulcerative colitis. By successfully combining dual-pathway inhibition (TNF and IL-23) with a localized oral delivery system, SOR102 represents a sophisticated next-generation approach to IBD therapy. If these findings are replicated in larger cohorts, SOR102 could become a cornerstone in the transition toward more convenient, targeted, and safer biological treatments for inflammatory bowel diseases.
Funding and Registration
This study was funded by Sorriso Pharmaceuticals. The trial is registered with ClinicalTrials.gov, number NCT06080048.
References
Jairath V, Danese S, D’Haens GR, Feagan BG, Peyrin-Biroulet L, Sands BE, et al. Safety and pharmacokinetics of SOR102, an oral bispecific inhibitor of TNF and interleukin-23 in healthy participants and patients with ulcerative colitis: a first-in-human, double-blind, randomised, placebo-controlled, phase 1 trial. Lancet Gastroenterol Hepatol. 2026 Jan;11(1):34-45. doi: 10.1016/S2468-1253(25)00296-1.