Highlight
- Mavacamten significantly reduces the long-term need for septal reduction therapy in severely symptomatic obstructive HCM patients.
- At 128 weeks, 80.5% of patients experienced at least one class improvement in New York Heart Association (NYHA) functional status; nearly half had at least two-class improvement.
- The treatment demonstrated sustained reduction in left ventricular outflow tract gradients with good overall tolerability.
- Approximately 90% of patients remained on long-term mavacamten, indicating good adherence and manageable safety profile.
Study Background and Disease Burden
Hypertrophic cardiomyopathy (HCM) is a genetically mediated cardiac disorder characterized by left ventricular hypertrophy, often with left ventricular outflow tract (LVOT) obstruction. Obstructive HCM leads to significant morbidity due to symptoms such as dyspnea, chest pain, syncope, and heart failure. The conventional management for severely symptomatic patients who do not respond to medical therapy often involves invasive septal reduction therapy (SRT) through surgical myectomy or alcohol septal ablation.
Despite advances, many patients remain symptomatic with limited options, and SRT, although effective, carries procedural risks and is not available universally. Mavacamten, a novel cardiac myosin inhibitor, targets the underlying hypercontractility in obstructive HCM and has shown promise in reducing LVOT gradients and improving symptoms. The VALOR-HCM trial initially demonstrated that mavacamten reduced the short-term need for SRT, but the longer-term efficacy and safety required evaluation.
Study Design
VALOR-HCM (NCT04349072) was a double-blind, randomized, placebo-controlled, multicenter trial conducted at 19 U.S. sites including adults with symptomatic obstructive HCM referred for SRT. Enrollment occurred from July 2020 to October 2021.
Patients initially randomized to mavacamten received up to 128 weeks of the drug; those randomized to placebo switched to mavacamten at week 16 for a total exposure of 112 weeks. Dose adjustments were individualized based on echocardiographic assessments of LVOT gradients and left ventricular ejection fraction (LVEF). The primary composite endpoint was the proportion of patients who underwent SRT or remained eligible for it according to guideline criteria at 128 weeks.
Key Findings
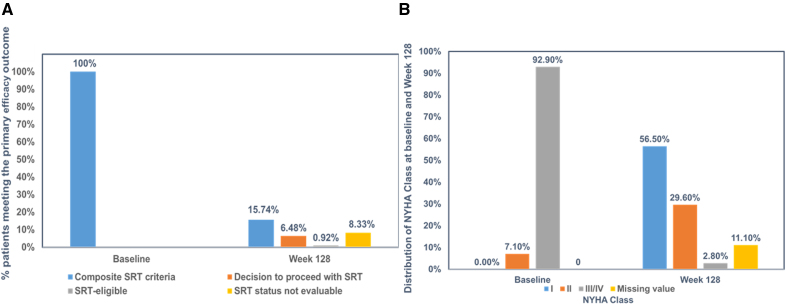
By week 128, only 15.7% (17 of 108) of patients reached the composite endpoint. This comprised 7 patients who underwent SRT, 1 who remained eligible, and 9 with unevaluable SRT status. This demonstrated sustained avoidance of invasive procedures for the majority.
Symptomatic improvement was robust: 80.5% (87/108) of patients improved by at least one NYHA functional class, and 48.1% (52/108) improved by two or more classes, signifying meaningful enhancement in daily activities and quality of life.
Primary and key efficacy parameters from baseline to week 128 in VALOR-HCM trial.
Mavacamten induced persistent reductions in resting LVOT gradients by a mean of 38.2 mm Hg and Valsalva maneuver gradients by 59.4 mm Hg, underscoring sustained hemodynamic benefit.
Regarding safety, 13.8% (15/108) of patients developed LVEF below 50%, including 2 patients with severely reduced LVEF (≤30%) and 1 reported death during the study. Notably, 80% of patients with reduced LVEF were able to continue mavacamten under medical supervision. New-onset atrial fibrillation occurred in 10.2% of patients, reflecting an incidence of 4.55 per 100 patient-years, a rate consistent with the natural history of HCM.
Importantly, 88% of participants transitioned to commercial mavacamten after trial completion, indicating acceptance and feasibility of long-term outpatient therapy.
Expert Commentary
The VALOR-HCM study extends pivotal evidence supporting mavacamten as an effective long-term medical therapy in obstructive HCM with severe symptoms, potentially altering the treatment paradigm. By targeting the molecular etiology of hypercontractility, mavacamten helps obviate or delay invasive interventions with durable symptomatic and hemodynamic improvements.
However, careful patient selection and monitoring for LV systolic dysfunction are critical given observed reductions in LVEF in a minority. The trial’s strengths include robust randomization, extended follow-up, and standardized dose titration but also highlight limitations such as the open-label extension for some patients and reliance on guideline criteria for SRT eligibility.
Future research may explore the role of mavacamten earlier in the disease course, its combination with other treatments, and its impact on long-term outcomes such as mortality and sudden cardiac death risk.
Conclusion
VALOR-HCM establishes that mavacamten offers a sustained, impactful therapy in severely symptomatic obstructive HCM patients, significantly reducing the necessity for septal reduction procedures over 2.5 years. The drug’s favorable efficacy, long-term adherence, and manageable safety underscore its potential to transform HCM management and improve patient quality of life. Adoption of mavacamten into clinical practice should incorporate comprehensive echocardiographic monitoring and strategic patient selection to maximize benefits and minimize risks.
References
Desai MY, Wolski K, Owens A, Geske JB, Saberi S, Wang A, Sherrid M, Cremer PC, Lakdawala NK, Tower-Rader A, Fermin D, Naidu SS, Smedira NG, Schaff H, Gong Z, Mudarris L, Lampl K, Sehnert AJ, Nissen SE; VALOR-HCM Investigators. Mavacamten in Patients With Hypertrophic Cardiomyopathy Referred for Septal Reduction: Week 128 Results From VALOR-HCM. Circulation. 2025 May 13;151(19):1378-1390. doi: 10.1161/CIRCULATIONAHA.124.072445 IF: 38.6 Q1 . Epub 2024 Nov 18. Erratum in: Circulation. 2025 May 13;151(19):e1001. doi: 10.1161/CIR.0000000000001339 IF: 38.6 Q1 . PMID: 39556124 IF: 38.6 Q1 ; PMCID: PMC12063683 IF: 38.6 Q1 .