Highlights
- LEAP-015 is the largest phase III trial to evaluate lenvatinib plus pembrolizumab and chemotherapy in advanced gastroesophageal adenocarcinoma.
- Combination therapy improved progression-free survival (PFS) and objective response rate (ORR) but did not significantly extend overall survival (OS).
- High rates of grade ≥3 adverse events observed with the combination regimen.
- No OS benefit in the PD-L1 CPS ≥1 subgroup, questioning the additive value of multi-modality first-line therapy.
Study Background and Disease Burden
Gastroesophageal adenocarcinoma (GEA), encompassing cancers of the stomach and gastroesophageal junction, represents a significant global health burden, with high mortality and limited treatment options for advanced and metastatic disease. Standard first-line therapy for unresectable, HER2-negative advanced GEA has traditionally centered on platinum-based chemotherapy. The integration of immunotherapy, specifically PD-1 inhibitors, has gradually altered the therapeutic landscape, yet optimal combinations and sequencing remain areas of active investigation. The LEAP-015 study addresses the clinical question of whether intensifying first-line treatment with lenvatinib (a multi-kinase inhibitor) and pembrolizumab (an anti-PD-1 antibody), in addition to chemotherapy, confers meaningful benefit for this population.
Study Design
LEAP-015 (ClinicalTrials.gov: NCT04662710) was an international, phase III, open-label, randomized controlled trial enrolling 880 adults (≥18 years) with untreated, HER2-negative, locally advanced unresectable or metastatic gastroesophageal adenocarcinoma. Participants were randomized 1:1 to:
- Experimental arm: Induction with lenvatinib 8 mg orally once daily, pembrolizumab 400 mg IV every 6 weeks (×2), and either capecitabine/oxaliplatin (every 3 weeks ×4) or fluorouracil/leucovorin/oxaliplatin (every 2 weeks ×6), followed by consolidation lenvatinib plus pembrolizumab.
- Control arm: Chemotherapy alone, with the same regimens and schedules, followed by continued chemotherapy.
The dual primary endpoints were progression-free survival (PFS) and overall survival (OS), assessed both in all participants and in the PD-L1 combined positive score (CPS) ≥1 subgroup. Secondary endpoints included objective response rate (ORR) and duration of response. Safety and tolerability were closely monitored.
Key Findings
- Total enrolled: 880 (443 experimental arm, 437 control arm).
- Median follow-up: ~32 months in both all-comers and PD-L1 CPS ≥1 groups.
Progression-Free Survival (PFS)
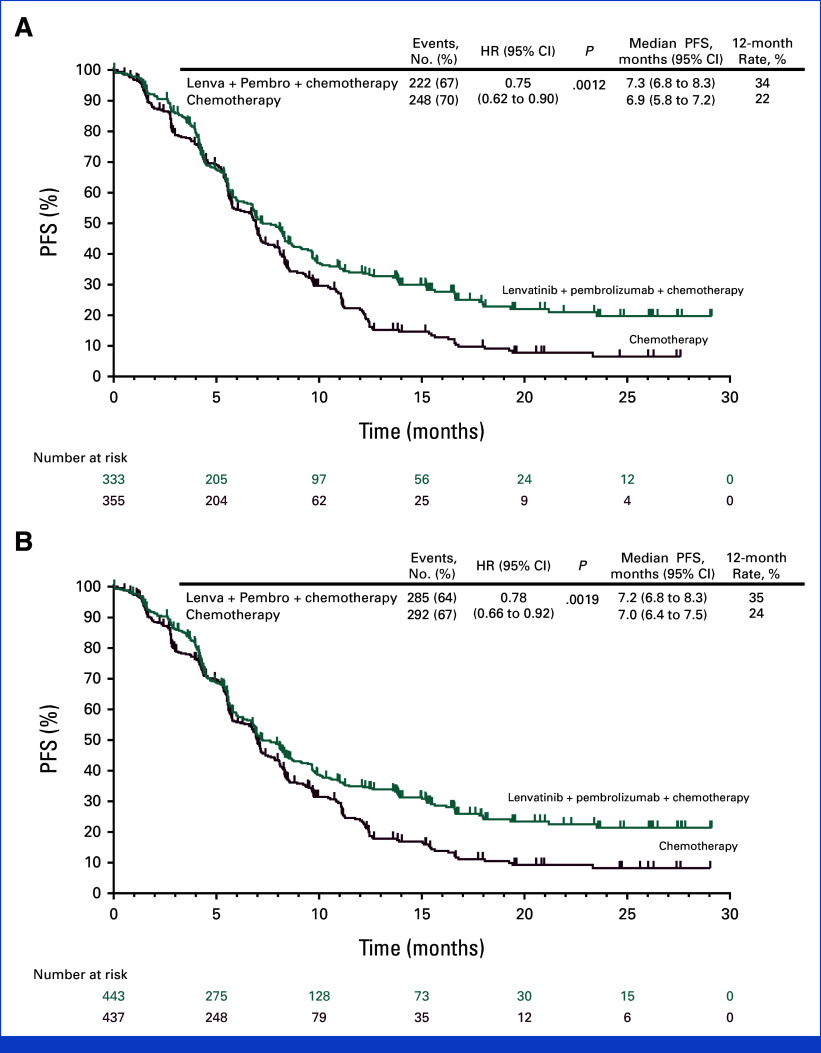
- PD-L1 CPS ≥1: Median PFS was 7.3 months (lenvatinib + pembrolizumab + chemo) vs 6.9 months (chemo); HR 0.75 (95% CI, 0.62–0.9); P = .0012.
- All participants: Median PFS was 7.2 vs 7.0 months; HR 0.78 (95% CI, 0.66–0.92); P = .0019.
Objective Response Rate (ORR)
- PD-L1 CPS ≥1: 59.5% (combination) vs 45.4% (chemo), P < .0001.
- All participants: 58.0% vs 43.9%, P < .0001.
Overall Survival (OS)
- PD-L1 CPS ≥1: Median OS was 12.6 months (combination) vs 12.9 months (chemo); HR 0.84 (95% CI, 0.71–1.00); P = .0244 (did not meet significance threshold, P boundary = .0204).
- All participants: OS data not provided in summary, but no significant OS improvement indicated.
Safety
- Grade ≥3 drug-related adverse events: 65% (combination) vs 49% (chemo only).
- No new safety signals, but increased toxicity with the addition of lenvatinib and pembrolizumab.
Expert Commentary
The LEAP-015 study demonstrates that the addition of lenvatinib and pembrolizumab to chemotherapy as first-line treatment for advanced GEA statistically improves PFS and ORR, but the absolute differences are modest. Critically, the lack of significant OS benefit, especially in the PD-L1 CPS ≥1 group, tempers enthusiasm for this regimen. The increased rate of grade ≥3 adverse events is also clinically relevant, potentially impacting patients’ quality of life and the feasibility of sustained therapy.
These findings align with the evolving paradigm in GEA management, where immunotherapy (notably pembrolizumab or nivolumab) is increasingly incorporated in the first-line setting for patients with PD-L1 positive tumors. However, the additive benefit of including a multi-kinase inhibitor such as lenvatinib remains questionable when weighed against toxicity and cost. As highlighted in recent expert editorials, future research should focus on biomarker-driven patient selection and optimizing combination strategies to maximize benefit while minimizing harm.
Conclusion
The LEAP-015 phase III trial confirms that, while lenvatinib plus pembrolizumab and chemotherapy can statistically prolong PFS and increase ORR in advanced unresectable or metastatic gastroesophageal adenocarcinoma, the incremental benefit is small and does not translate into a significant OS advantage. The high toxicity burden further limits the routine clinical adoption of this triple regimen. Personalized treatment approaches and improved biomarkers are needed to identify patients who may derive true benefit from intensified combination therapy.
References
- Shitara K, Lorenzen S, Li J, et al. Lenvatinib Plus Pembrolizumab and Chemotherapy Versus Chemotherapy in Advanced Metastatic Gastroesophageal Adenocarcinoma: The Phase III, Randomized LEAP-015 Study. J Clin Oncol. 2025 Aug;43(22):2502-2514. doi: 10.1200/JCO-25-00748 IF: 41.9 Q1 . PMID: 40448579 IF: 41.9 Q1 ; PMCID: PMC12288889 IF: 41.9 Q1 .PDF (1.2 MB)
- Kelly RJ, Ajani JA, Kuzdzal J, et al. Nivolumab plus chemotherapy in advanced esophageal squamous-cell carcinoma. N Engl J Med. 2022;386(5):449-462.
- Janjigian YY, Shitara K, Moehler M, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. 2021;398(10294):27-40.