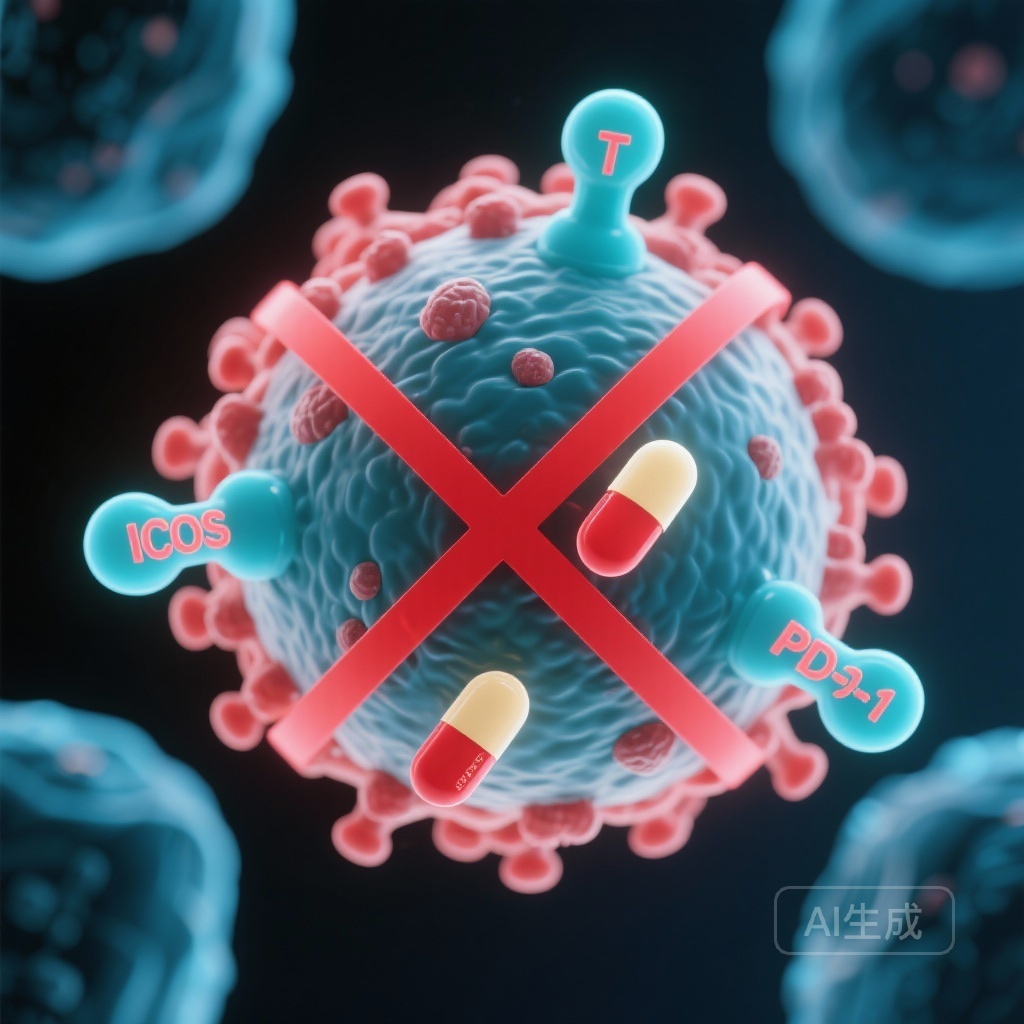
The Failed Promise of ICOS Agonism: Lessons from the INDUCE-3 Trial
The therapeutic landscape for recurrent or metastatic head and neck squamous cell carcinoma (HNSCC) has been transformed by the introduction of programmed cell death protein 1 (PD-1) inhibitors. Following the landmark KEYNOTE-048 trial, pembrolizumab, either as a monotherapy for PD-L1-expressing tumors or in combination with platinum-based chemotherapy, has become the standard of care for first-line treatment. However, despite these advances, many patients do not achieve durable responses, prompting the search for combination therapies that can enhance immune activation. One such strategy involved targeting the Inducible T-cell Co-stimulatory (ICOS) receptor. Feladilimab, an ICOS agonist, initially showed promise in early-phase studies, but the results of the definitive Phase II/III INDUCE-3 trial have recently challenged the viability of this combination.
Highlights
- The INDUCE-3 trial was halted early following an interim analysis that demonstrated no efficacy benefit for the combination of feladilimab and pembrolizumab.
- The hazard ratio for overall survival (OS) was 1.51, and for progression-free survival (PFS) was 1.40, both favoring the pembrolizumab monotherapy (placebo) arm.
- Median PFS was significantly shorter in the combination arm (10.1 weeks) compared to the placebo arm (16.0 weeks).
- These findings underscore the complexity of co-stimulatory pathways and the difficulty of translating Phase I expansion signals into Phase III success.
Background and Clinical Context
HNSCC is a biologically aggressive malignancy with a high disease burden and a propensity for recurrence. For years, platinum-based chemotherapy remained the only option, but the advent of immunotherapy offered a new mechanism to exploit the immune system’s ability to recognize and destroy tumor cells. ICOS is a member of the CD28 family of co-stimulatory molecules, primarily expressed on activated T cells. Preclinical data suggested that ICOS agonism could synergize with PD-1 blockade by promoting the expansion of effector T cells and reducing the suppressive activity of regulatory T cells (Tregs).
Early clinical data from the INDUCE-1 trial, specifically a Phase I expansion cohort of HNSCC patients, provided the signal necessary to launch INDUCE-3. The goal was to determine if feladilimab could turn ‘cold’ tumors ‘hot’ or further potentiate the efficacy of pembrolizumab in patients whose tumors expressed PD-L1.
Study Design and Methodology
INDUCE-3 (NCT04128696) was designed as a randomized, double-blind, Phase II/III study. It utilized an innovative ‘2-in-1’ adaptive design, which allowed for a seamless transition from a Phase II efficacy screening to a Phase III confirmatory trial if specific criteria were met. The study population consisted of patients with first-line recurrent and/or metastatic HNSCC whose tumors were PD-L1 positive (Combined Positive Score [CPS] ≥ 1).
A total of 315 patients were enrolled and randomized 1:1 to receive either:
1. Feladilimab (an ICOS agonist) plus pembrolizumab.
2. Placebo plus pembrolizumab.
Treatment was planned for up to 35 cycles, lasting approximately two years. The primary endpoints were overall survival (OS) and investigator-assessed progression-free survival (PFS) in the intent-to-treat population.
Key Findings: A Critical Analysis of Efficacy and Safety
The trial’s progress was interrupted when an Independent Data Monitoring Committee (IDMC) reviewed unblinded interim data from the first 140 patients. The results were starkly negative, leading to the immediate cessation of patient accrual based on prespecified futility criteria.
Survival Outcomes
The statistical analysis revealed no evidence of benefit for the feladilimab-pembrolizumab combination. In fact, the data suggested a trend toward worse outcomes for those receiving the experimental agonist. The adjusted hazard ratio (HR) for OS was 1.51, indicating a higher risk of death in the combination arm. The median OS for the combination group was 44.1 weeks (95% CI: 35.9-NA), while the median OS for the placebo plus pembrolizumab group was not reached at the time of the analysis.
PFS data mirrored the OS findings. The adjusted HR for PFS was 1.40. The median PFS for patients receiving feladilimab plus pembrolizumab was only 10.1 weeks (95% CI: 9.1-15.0), compared to 16.0 weeks (95% CI: 14.3-26.1) in the placebo plus pembrolizumab group. This disparity highlights a lack of additive or synergistic effect, and potentially even an antagonistic interaction between the two agents or a negative impact of ICOS stimulation in this specific clinical context.
Safety and Adverse Events
Interestingly, the study reported that the incidence of treatment-related adverse events (TRAEs) was higher in the placebo group than in the group receiving feladilimab. While this might suggest that feladilimab did not add significant systemic toxicity, the early termination and shorter duration of treatment in the combination arm (due to rapid progression) may have influenced the reporting of cumulative toxicities. Regardless, the safety profile was not the primary driver for stopping the trial; the lack of efficacy was the decisive factor.
Expert Commentary and Mechanistic Insights
The failure of INDUCE-3 is a significant setback for the development of ICOS-targeted therapies. Clinicians and researchers must now grapple with why a biological rationale that seemed robust in the lab and in early-phase cohorts failed to materialize in a larger randomized setting. Several hypotheses exist:
1. The Agonist vs. Antagonist Paradox
While ICOS is a co-stimulatory receptor on effector T cells, it is also highly expressed on regulatory T cells. It is possible that feladilimab, despite being designed as an agonist, may have inadvertently stimulated suppressive immune populations, thereby dampening the anti-tumor response of pembrolizumab.
2. Optimal Dosing and Receptor Occupancy
Agonistic antibodies are notoriously difficult to dose. Unlike antagonistic antibodies (like PD-1 inhibitors), where the goal is simply to block a receptor, agonists must achieve a specific level of receptor clustering and signaling without inducing receptor internalization or T-cell exhaustion. It is possible that the dosing schedule used in INDUCE-3 did not achieve the narrow therapeutic window required for true co-stimulation.
3. Patient Heterogeneity
HNSCC is a heterogeneous disease. While the trial selected for PD-L1 expression, other biomarkers—such as the presence of specific T-cell subsets or the metabolic state of the tumor microenvironment—may be necessary to identify the small subset of patients who might truly benefit from ICOS agonism.
Conclusion
The INDUCE-3 trial provides definitive evidence that the addition of feladilimab to pembrolizumab does not improve survival or progression-free outcomes in the first-line treatment of R/M HNSCC. Following the recommendation of the IDMC, the combination is no longer being pursued in this setting. This study serves as a cautionary tale in oncology drug development, highlighting the risks of expanding into large-scale Phase III trials based on early signals from single-arm expansion cohorts. For now, pembrolizumab monotherapy or combination with chemotherapy remains the standard of care, while the search for the next meaningful immunotherapy partner continues through different pathways.
Funding and clinicaltrials.gov
The INDUCE-3 study was funded by GSK. ClinicalTrials.gov Identifier: NCT04128696.
References
Rischin D, Hansen AR, Cohen EEW, et al. INDUCE-3: A randomized Phase II/III study of first-line feladilimab plus pembrolizumab in patients with recurrent/metastatic head and neck squamous cell carcinoma. Clin Cancer Res. 2025 Dec 22. doi: 10.1158/1078-0432.CCR-25-1197. Epub ahead of print. PMID: 41427951.