Highlight
Unprecedented Progression-Free Survival
The addition of epcoritamab to the standard lenalidomide and rituximab (R2) regimen resulted in a 79% reduction in the risk of disease progression or death compared to R2 alone (Hazard Ratio 0.21).
Superior Response Rates
The combination achieved an overall response rate (ORR) of 95%, significantly higher than the 79% observed with the R2 control arm, demonstrating high efficacy in a difficult-to-treat population.
Manageable Safety Profile
Despite a higher incidence of Grade 3 or higher adverse events in the epcoritamab group, cytokine release syndrome (CRS) events were primarily low-grade (Grade 1-2) and all were successfully resolved, supporting the regimen’s use in outpatient or community settings.
The Evolving Landscape of Follicular Lymphoma
Follicular lymphoma (FL) remains the most common indolent non-Hodgkin lymphoma. While the disease is characterized by a high initial response to chemoimmunotherapy, it is also defined by a pattern of recurrent relapses, with each subsequent line of therapy often yielding shorter durations of remission. For patients who experience disease progression within 24 months of initial therapy (POD24) or those who are refractory to multiple lines of treatment, the clinical prognosis has historically been poor.
In recent years, the shift toward chemotherapy-free regimens has gained significant momentum. The combination of lenalidomide, an immunomodulatory agent, and rituximab, an anti-CD20 monoclonal antibody (the R2 regimen), established itself as a standard of care for relapsed or refractory (R/R) FL following the results of the AUGMENT trial. However, there remains a critical need for more potent combinations that can induce deeper and more durable responses without the cumulative toxicities associated with traditional cytotoxic chemotherapy.
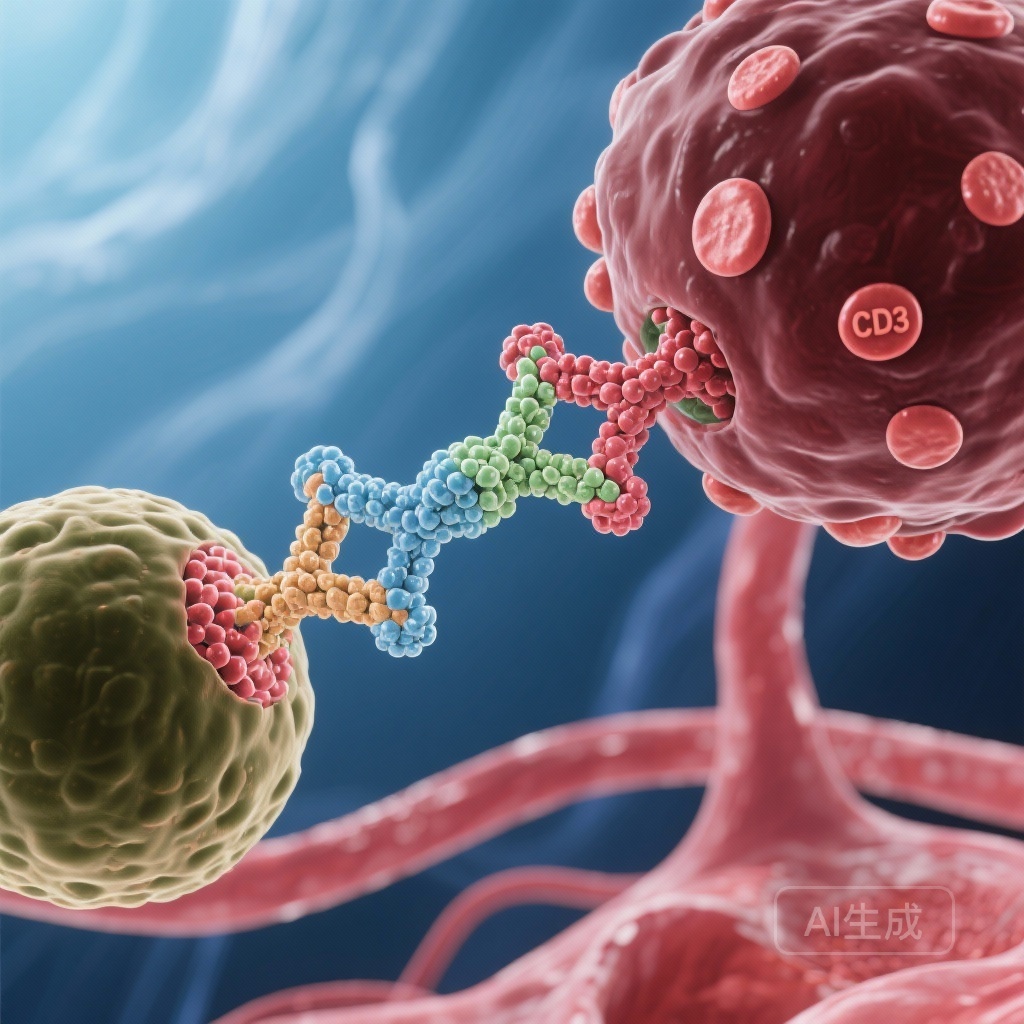
The Biological Rationale: Epcoritamab and T-Cell Engagement
Epcoritamab is a first-in-class, subcutaneous bispecific antibody that targets CD3 on T-cells and CD20 on B-cells. Its mechanism of action involves the redirection of endogenous cytotoxic T-cells to specifically eliminate CD20-positive malignant B-cells. Unlike CAR-T cell therapies, which require complex manufacturing and patient-specific engineering, epcoritamab is an “off-the-shelf” immunotherapy that can be administered more readily in various clinical settings.
The rationale for combining epcoritamab with R2 is synergistic. Lenalidomide enhances T-cell and natural killer (NK) cell activity, potentially augmenting the T-cell-mediated cytotoxicity induced by epcoritamab. Rituximab provides additional B-cell depletion through antibody-dependent cellular cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC). Together, this triple-combination approach aims to maximize the immune system’s ability to eradicate lymphoma cells.
EPCORE FL-1: Study Design and Participant Characteristics
The EPCORE FL-1 trial was a global, open-label, randomized, phase 3 trial conducted across 189 centers in 30 countries. The study enrolled 488 participants with R/R follicular lymphoma who had received at least one prior line of chemoimmunotherapy. Participants were randomly assigned in a 1:1 ratio to receive either epcoritamab plus R2 or R2 alone for up to 12 cycles.
The epcoritamab dosing schedule was designed to mitigate the risk of cytokine release syndrome, utilizing a step-up dosing approach in the first cycle, followed by weekly administration in cycles 1-3 and every four weeks in cycles 4-12. Lenalidomide was administered daily (days 1-21 of each 28-day cycle), and rituximab was given weekly in cycle 1 and monthly in cycles 2-5. The dual primary endpoints were the overall response rate (ORR) and progression-free survival (PFS) as determined by an independent review committee (IRC).
Efficacy Outcomes: A Paradigm Shift in PFS
The trial met its primary endpoints with remarkable statistical and clinical significance. At a median follow-up of 14.8 months, the ORR was 95% (95% CI 92-97) for the epcoritamab plus R2 group compared to 79% (95% CI 74-84) for the R2 control group (p<0.0001). This high rate of response suggests that the addition of epcoritamab can overcome resistance mechanisms prevalent in R/R follicular lymphoma.
More importantly, the progression-free survival data revealed a substantial benefit. The hazard ratio (HR) for progression or death was 0.21 (95% CI 0.14-0.31, p<0.0001), indicating that the epcoritamab combination reduced the risk of disease progression by nearly 80%. The estimated 16-month PFS rate was 85.5% in the epcoritamab-R2 arm compared to just 40.2% in the R2 arm. These results are particularly noteworthy given that the control arm (R2) performed consistently with historical benchmarks, highlighting the potency of the experimental triple therapy.
Safety Analysis: Managing the Immunotherapy Profile
As expected with the addition of a potent immunotherapy to an existing regimen, the incidence of adverse events (AEs) was higher in the epcoritamab plus R2 group. Grade 3 or higher AEs occurred in 90% of participants receiving the triple combination, compared to 68% in the R2 group. The most common high-grade AEs were hematological, including neutropenia, which is consistent with the known safety profile of lenalidomide.
A key focus for bispecific antibodies is the incidence and severity of cytokine release syndrome (CRS). In the EPCORE FL-1 trial, CRS occurred in 26% of patients in the epcoritamab-R2 arm. Crucially, all CRS events were low-grade (21% Grade 1 and 5% Grade 2), and no Grade 3 or higher CRS events were reported. All cases were manageable and resolved with standard protocols, including the use of tocilizumab and corticosteroids when necessary. The low incidence of severe CRS and the subcutaneous administration route suggest that this regimen could be feasible for broader clinical use outside of major academic transplant centers.
Clinical Implications and Expert Commentary
The results of EPCORE FL-1 represent a significant milestone in the treatment of follicular lymphoma. By achieving a hazard ratio of 0.21 in a Phase 3 trial, the epcoritamab plus R2 regimen has set a new efficacy benchmark for second-line therapy and beyond. The findings suggest that early integration of T-cell engaging therapies can provide deep, durable remissions that were previously difficult to achieve without intensive chemotherapy or autologous stem cell transplantation.
Experts in the field note that the high response rate and PFS benefit observed in this trial may challenge the current sequencing of therapies. While CAR-T cell therapies offer high efficacy, they are often reserved for later lines of treatment due to logistical complexities and toxicity concerns. Epcoritamab-R2 provides a highly effective, off-the-shelf alternative that can be administered earlier in the disease course, potentially improving the long-term outlook for patients with high-risk features like POD24.
However, clinicians must remain vigilant regarding the management of cumulative toxicities and the increased risk of infections associated with prolonged B-cell depletion. The 12-cycle fixed-duration approach used in this trial is an important design element, as it attempts to balance maximum therapeutic benefit with the need for treatment-free intervals and immune recovery.
Conclusion
The EPCORE FL-1 trial provides robust evidence that epcoritamab plus R2 is superior to R2 alone in patients with relapsed or refractory follicular lymphoma. The combination offers a highly effective, chemotherapy-free option that significantly extends progression-free survival while maintaining a manageable safety profile. These findings position epcoritamab plus R2 as a new standard of care for second-line or subsequent treatment of follicular lymphoma, potentially transforming the management of this chronic and relapsing malignancy.
Funding and Clinical Trial Information
The EPCORE FL-1 trial was funded by AbbVie and Genmab. The study is registered with ClinicalTrials.gov (NCT05409066) and EudraCT (2021-000169-34). The trial is currently ongoing, though it is closed to recruitment.
References
Falchi L, Nijland M, Huang H, et al. Epcoritamab, lenalidomide, and rituximab versus lenalidomide and rituximab for relapsed or refractory follicular lymphoma (EPCORE FL-1): a global, open-label, randomised, phase 3 trial. Lancet. 2026;407(10524):161-173. doi:10.1016/S0140-6736(25)02360-8.
Leonard JP, Trneny M, Izutsu K, et al. AUGMENT: A Phase III Study of Lenalidomide Plus Rituximab Versus Placebo Plus Rituximab in Relapsed or Refractory Indolent Lymphoma. J Clin Oncol. 2019;37(14):1188-1199.