Introduction
Circular RNAs (circRNAs) represent a class of covalently closed-loop non-coding RNAs that have emerged as critical regulators in numerous biological processes, notably in cancer and immune modulation. With their unique stable structure and ability to interact intricately with microRNAs (miRNAs), RNA-binding proteins (RBPs), and the translational machinery, circRNAs influence tumor progression and the tumor microenvironment (TME). Their promising roles as biomarkers and therapeutic targets, particularly in cancer immunotherapy, demand a comprehensive understanding of their biogenesis, functions, and translational potential.
Biogenesis and Functional Mechanisms of CircRNAs
CircRNAs are predominantly produced through a back-splicing mechanism of precursor messenger RNA (pre-mRNA), wherein a downstream splice donor site joins with an upstream splice acceptor site forming a closed circular RNA. Their generation is dynamically regulated by transcription elongation rates and modulated by intron complementary sequences and trans-acting factors including RBPs such as Quaking (QKI) and HuR. Nuclear export of circRNAs depends on their length and interaction with RNA helicases (DDX39A/B), followed by translocation through the nuclear pore complex facilitated by NXT1-NXF1 heterodimers.
Functionally, circRNAs act via multiple validated and putative mechanisms:
1. **miRNA Sponge:** CircRNAs contain multiple binding sites for specific miRNAs, enabling them to sequester miRNAs and relieve repression on their target mRNAs. This is exemplified by CDR1as, which sequesters miR-7 to modulate oncogene expression such as EGFR.
2. **RBP Interaction:** CircRNAs bind RBPs, impacting their own stability, nuclear retention, and biogenesis, as well as modulating gene expression indirectly. CircPABPN1’s interaction with HuR exemplifies this mechanism.
3. **Protein Scaffolding:** Certain circRNAs serve as platforms assembling multiple proteins, influencing cell cycle progression and apoptosis—as seen with circFoxo3 complexing with p21 and CDK2.
4. **Translation Template:** Emerging evidence suggests translation of circRNAs into functional peptides via Internal Ribosome Entry Sites (IRES), adding a novel layer to gene regulation, though this remains an area requiring further validation.
CircRNAs in Immune Modulation and Tumor Microenvironment
CircRNAs modulate immune responses intricately within the TME by affecting various immune cells and checkpoints:
– **T Cells:** CircRNA-derived cryptic peptides are processed and presented on tumor cells by MHC class I molecules, eliciting CD8+ cytotoxic T cell responses and cytokine secretion (IFN-γ, TNF-α), enhancing antitumor immunity.
– **Natural Killer (NK) Cells:** CircPHLPP2 suppresses NK cell infiltration and cytotoxic function in colorectal cancer by modulating IL36γ transcription, contributing to immune evasion.
– **Macrophage Polarization:** Exosome-mediated delivery of circZNF451 to macrophages skews them towards the immunosuppressive M2 phenotype, impeding antitumor immunity.
– **Immune Checkpoints:** CircRNAs regulate the expression of PD-L1 and CTLA-4 by sponging miRNAs that target these immune checkpoint mRNAs, thus facilitating tumor immune escape.
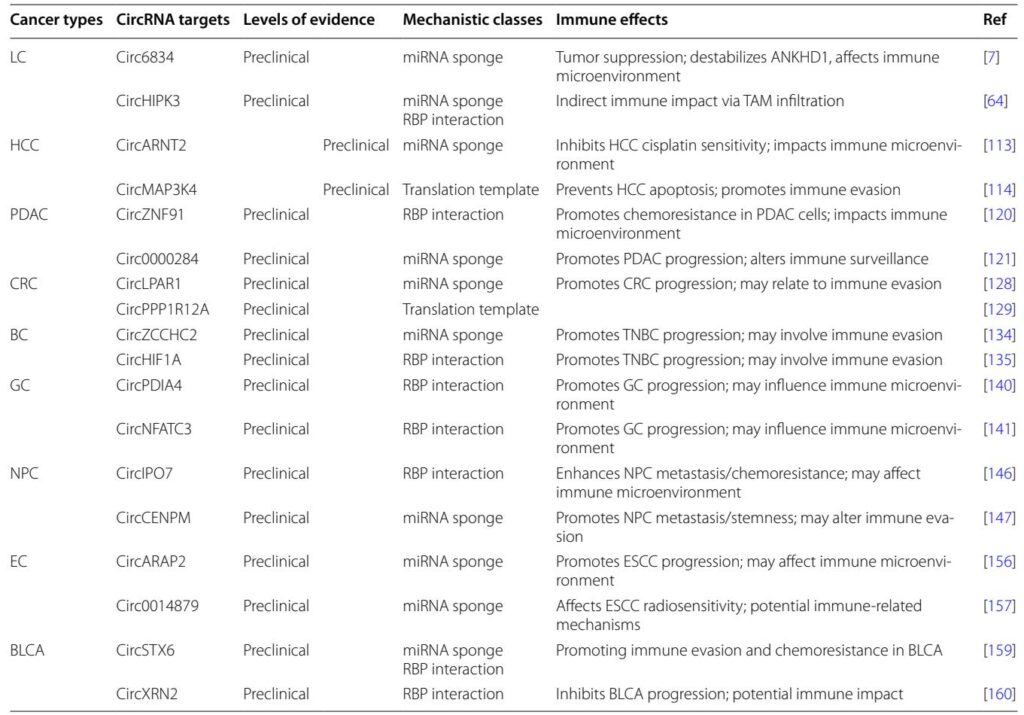
Roles of CircRNAs in Specific Cancers
Extensive preclinical studies have characterized circRNAs across multiple malignancies:
– **Lung Cancer:** Circ6834 and circHIPK3 regulate tumor progression and macrophage infiltration, respectively, impacting the immune milieu.
– **Hepatocellular Carcinoma:** CircARNT2 impairs cisplatin sensitivity via miRNA sponging, whereas circMAP3K4 encodes a peptide preventing apoptosis.
– **Pancreatic Ductal Adenocarcinoma:** CircZNF91 promotes chemoresistance by RBP-mediated mechanisms; circ_0000284 fosters tumor growth via miRNA interaction.
– **Colorectal Cancer:** CircLPAR1 and circPPP1R12A promote proliferation and metastasis via miRNA sponging and coding for oncogenic peptides.
– **Breast and Gastric Cancers:** Numerous circRNAs participate in tumor progression and immune evasion via diverse mechanisms including RBP binding and miRNA sponge activity.
Further cancers including nasopharyngeal carcinoma, esophageal cancer, and bladder urothelial carcinoma also show circRNA involvement in tumor biology and immune modulation, predominantly at preclinical stages.
Emerging Therapeutic Applications
– **CAR-T Cell Therapy:** Leveraging circRNA enhanced stability, engineered circRNAs incorporated into CAR-T cells aim to improve persistence, specificity, and efficacy, especially against solid tumors. Despite promising preclinical data, challenges with tumor penetration and T cell exhaustion remain.
– **CircRNA-Based Vaccines:** CircRNA vaccines show improved stability and prolonged antigen presentation compared to linear mRNA vaccines, inducing robust dendritic and CD8+ T cell responses in animal cancer models. Novel cancer neoantigens derived from circRNA translation expand vaccine targets.
– **CircRNA Databases:** Resources such as MiOncoCirc and TCCIA integrate circRNA profiles with immunotherapy responses, facilitating biomarker discovery and therapeutic target identification. Technical and validation challenges persist.
Challenges and Future Directions
The translation of circRNA biology into clinical applications faces significant hurdles. These include incomplete understanding of circRNA biogenesis and degradation, delivery system inefficiencies, potential immunogenicity, and off-target effects. Scaling manufacturing, rigorous preclinical safety assessments, and expansive clinical trials are necessary. Combining circRNA vaccines with CAR-T therapies may synergistically enhance antitumor immunity. Standardization of detection methods and multi-omic integration will accelerate discovery and translation.
Conclusion
CircRNAs represent a versatile, multifunctional class of regulatory RNAs with significant implications in cancer progression and immunotherapy. Through diverse mechanisms including miRNA sponging, protein interactions, and neoantigen generation, circRNAs modulate tumor immunity and the TME. While promising as biomarkers and therapeutic agents, clinical translation mandates overcoming technical and biological challenges. Future research optimizing delivery, elucidating mechanistic pathways, and integrating circRNA-based modalities holds promise for advancing cancer immunotherapy.
References
Hansen TB, Jensen TI, Clausen BH, Bramsen JB, Finsen B, Damgaard CK, et al. Natural RNA circles function as efcient microrna sponges. Nature. 2013;495(7441):384–8.
Yan Wang et al., CircRNAs: functions and emerging roles in cancer and immunotherapy. Mol Cancer. 2025; [As detailed in the provided article].