Highlights
– Multimodal phenotyping of two pig-to-human heart xenografts identified early endothelial activation and mild microvascular inflammation dominated by innate immune cells (CD15+ neutrophils and CD68+ monocytes/macrophages).
– Gene-expression profiling showed upregulation of pathways linked to monocyte/macrophage activation, neutrophil activation, interferon-γ response, NK-cell burden, endothelial activation, apoptosis, and repair — a molecular pattern reminiscent of antibody-mediated rejection despite absent capillary C4d.
– The observed phenotype was not present in multiple control pig hearts, including wild-type hearts subjected to ischemia/reperfusion and brain death, supporting a xenogeneic-specific host response.
– A multimodal, precision diagnostic platform (morphology, immunophenotyping, ultrastructure, automated multiplex IF, and molecular profiling) can enhance early detection and mechanistic understanding of xenoimmune injury and guide targeted interventions.
Background
Heart failure remains a major cause of morbidity and mortality worldwide, and donor shortage is a critical barrier to life-saving transplantation. Genetically engineered porcine organs have emerged as the leading near-term solution to organ scarcity. Recent clinical-grade advances, including multigene-edited donor pigs and improved perioperative care, culminated in the first reported pig-to-human heart xenotransplants. Despite these milestones, the character and timing of the human immune response to porcine cardiac tissue — the xenoimmune response — remain incompletely defined. Understanding early tissue-level events after reperfusion is essential to distinguish expected ischemia/reperfusion (I/R) changes from true rejection, to optimize monitoring, and to design appropriately targeted immunomodulatory strategies.
Study design
Objective
To characterize histologic, cellular, ultrastructural, and transcriptional signatures of early xenoimmune injury in pig-to-human heart xenografts using an integrated, multimodal diagnostic pipeline.
Subjects and samples
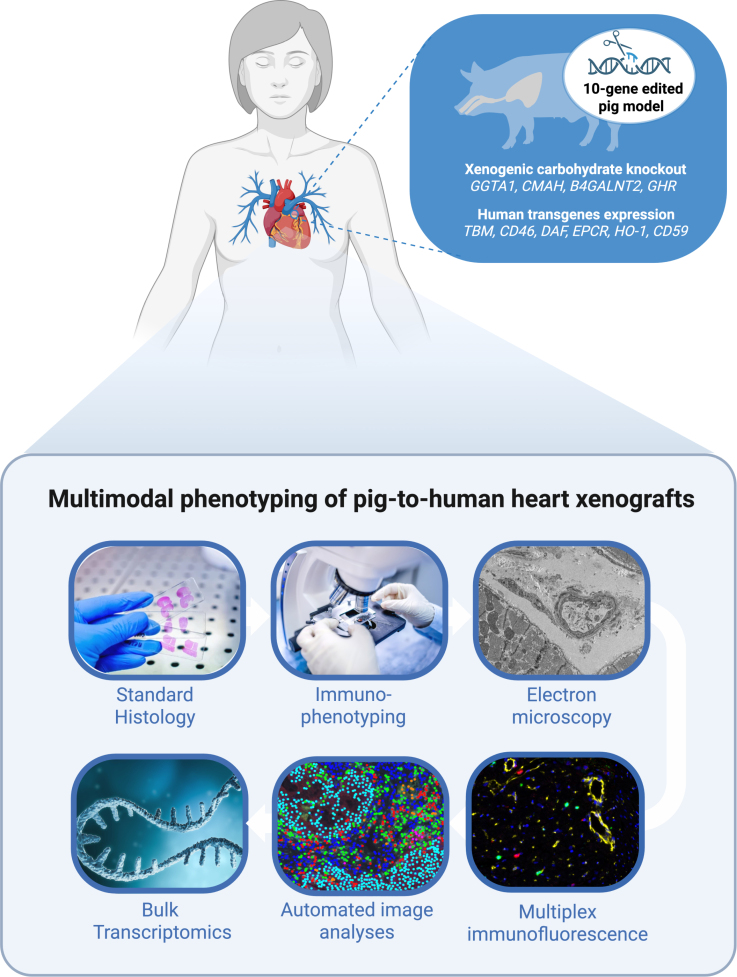
Two orthotopic pig-to-human heart xenografts, from 10-gene–edited donor pigs, were transplanted into brain-dead human recipients. Myocardial biopsy specimens were collected 66 hours after reperfusion. Control tissues included the donor xenografts sampled before implantation and multiple wild-type pig hearts obtained under conditions with or without brain death and with or without ischemia/reperfusion injury.
Methods
A multimodal phenotyping approach was applied to the 66-hour biopsies: conventional histopathology, immunophenotyping, electron microscopy (ultrastructure), deep learning–enabled automated quantification of multiplex immunofluorescence (mIF), and gene expression profiling (bulk/transcriptomic). The combination enabled correlation across morphology, cell-type composition, microanatomy, and molecular pathways.
Key findings
1. Morphology and microvascular inflammation
Both xenograft biopsies exhibited endothelial activation and mild microvascular inflammation. Classic capillary deposition of C4d, a canonical marker of complement activation and antibody-mediated rejection in allografts, was absent. Light microscopy revealed focal microvascular leukocyte accumulation without widespread myocyte necrosis at this early time point.
2. Cellular composition: innate immune predominance
Immunophenotyping and automated mIF quantification identified CD15+ cells (predominantly neutrophils) and CD68+ cells (monocytes/macrophages) as the major inflammatory cell types within microvessels and perivascular spaces. Lymphocyte populations (CD3+ T cells, CD20+ B cells) were comparatively sparse at 66 hours.
3. Ultrastructural features
Electron microscopy demonstrated endothelial swelling and endothelial cell activation with occasional intravascular leukocytes adherent to or marginally transmigrating across the endothelium. No consistent ultrastructural evidence of widespread complement-mediated membrane attack complexes was reported at this time point.
4. Molecular signature consistent with antibody-mediated and innate activation
Transcriptomic profiling revealed upregulation of gene sets associated with monocyte/macrophage activation, neutrophil degranulation/activation, interferon-γ response, NK-cell burden, endothelial activation, apoptosis, and tissue repair pathways. These pathways collectively resemble molecular patterns described in antibody-mediated rejection (AMR) in human allografts, despite the histologic absence of C4d.
5. Controls did not replicate the xenograft phenotype
All control pig hearts — including wild-type hearts subjected to brain death and ischemia/reperfusion injury — lacked the composite molecular and cellular signature observed in the xenografts. This argues that the observed phenotype is linked to species disparity and/or the host response to porcine antigens rather than nonspecific transplantation-related injury.
6. Interpretative synthesis
Taken together, the multimodal data describe an early, predominantly innate xenoimmune response featuring endothelial activation and microvascular inflammation dominated by neutrophils and macrophages. The molecular pattern resembles AMR, implying that humoral mechanisms, Fc-receptor–mediated effector functions, complement-independent antibody pathways, or a mixed innate–humoral interaction may be operative early after xenotransplantation.
Expert commentary and mechanistic considerations
The findings are biologically plausible and align with preclinical xenotransplantation work showing prominent innate immune involvement early after reperfusion. Key mechanistic points:
– Endothelial activation is a sentinel event. Endothelial cells express species-specific carbohydrate and protein antigens that can be recognized by preformed natural antibodies and by induced humoral responses. Even in the absence of classical complement deposition (C4d), antibodies can drive Fc-mediated activation of myeloid cells, or trigger complement-independent endothelial signaling leading to upregulation of adhesion molecules and chemokines that recruit neutrophils and monocytes.
– Innate cells as effectors. Neutrophils and monocyte-derived macrophages are rapid responders to tissue injury and foreign antigens. They can mediate microvascular obstruction, reactive oxygen species release, and cytokine production that amplify graft injury and recruit adaptive effectors over time.
– Molecular AMR signature without C4d. The combination of interferon-γ response, NK-cell burden, and macrophage activation is characteristic of antibody-mediated processes in which donor-specific antibodies (DSA) act via Fc receptors or through complement-independent mechanisms. Serial time-course studies will be needed to clarify whether complement activation becomes more prominent later.
Limitations to consider:
– Small sample size (two xenografts) and a single early timepoint (66 hours) limit generalizability and temporal interpretation.
– Recipients were brain-dead, a physiological state that can alter systemic inflammation and hemodynamics; this may accentuate or modify components of the observed response compared with living recipients.
– The donor pigs had 10-gene edits; specific gene-edit combinations can shape antigenicity and innate immune interactions. The study reports the edits but the relative contribution of each to the observed phenotype remains unknown.
Clinical and translational implications
1. Diagnostic strategy: The study demonstrates the value of integrating conventional histology with quantitative mIF and molecular profiling to detect early xenoimmune signatures that might be missed by single-modality assessment. For future clinical xenotransplant programs, standardized, multimodal surveillance (including protocol biopsies at early time points and targeted molecular panels) could improve early detection and mechanistic categorization of injury.
2. Therapeutic targets: Findings highlight potential targets beyond standard T-cell–directed immunosuppression: strategies to blunt innate myeloid recruitment/activation (macrophage inhibitors, CXCR2 pathway blockade for neutrophils), modulation of Fc receptor signaling, and endothelial stabilizers (anti-inflammatory endothelial modulators, complement inhibitors if complement activation emerges later). The early prominence of innate pathways suggests that perioperative regimens addressing innate immunity may be critical.
3. Biomarkers: The molecular signature components identified here could inform development of noninvasive biomarkers (plasma signatures, donor-derived cell-free DNA patterns, or serum cytokines) to complement tissue surveillance and to reduce reliance on invasive biopsies.
4. Research priorities: Key next steps include serial sampling in living recipients, correlation with circulating DSAs and complement activation products, and controlled comparisons across different gene-edit combinations to map which edits materially affect the early xenoimmune cascade.
Conclusion
This detailed, multimodal analysis of two pig-to-human heart xenografts at 66 hours post-reperfusion documents an early xenoimmune phenotype featuring endothelial activation and mild, innate cell–dominant microvascular inflammation, accompanied by a transcriptional program reminiscent of antibody-mediated injury. The phenotype was distinct from control pig hearts exposed to ischemia/reperfusion and brain death, supporting a xenogeneic-specific host response. These observations underscore the need for precision diagnostics that integrate histology, advanced immunophenotyping, ultrastructure, and molecular profiling in clinical xenotransplantation programs, and they point to innate immune and endothelial-directed interventions as important complements to conventional immunosuppression.
Funding and clinicaltrials.gov
Funding and trial registration details are reported by the authors in the primary publication (Giarraputo et al., Circulation 2025). No clinicaltrials.gov identifier is provided in the present summary; readers should consult the original article for full funding, conflicts of interest, and regulatory information.
Reference
Giarraputo A, Morgand E, Stern J, Mezine F, Coutance G, Goutaudier V, Sannier A, Certain A, Hauet T, Giraud S, Kerforne T, Allain G, Ayares D, Khalil K, Kim J, Mehta S, Narula N, Reyentovich A, Smith D, Tissier R, Saraon T, Kadosh B, Divita M, Goldberg R, Pass H, Mangiola M, Bruneval P, Griesemer A, Moazami N, Montgomery RA, Loupy A. Characterizing the Immune Response in Pig-to-Human Heart Xenografts Using a Multimodal Diagnostic System. Circulation. 2025 Dec 2;152(22):1552-1563. doi: 10.1161/CIRCULATIONAHA.125.074971 IF: 38.6 Q1 . Epub 2025 Oct 2. PMID: 41036838 IF: 38.6 Q1 ; PMCID: PMC12655869 IF: 38.6 Q1 .