Highlights
– In HFrEF, a steeper decline in estimated glomerular filtration rate (eGFR) can be detected up to 12 months before a heart-failure (HF) hospitalization or HF death.
– Patients who experienced HF events had pre-event eGFR slopes approximately -4 to -6 mL/min/1.73 m²/year versus ~-1 to -1.4 mL/min/1.73 m²/year in those without events across trial and real-world cohorts.
– eGFR continued to decline in the year after an HF event but at a slower rate, linking pre-event renal deterioration with symptomatic congestion and adverse outcomes.
– Serial eGFR trends (slopes) may identify at-risk patients earlier than isolated threshold-based changes and could inform intensification of decongestive therapy, monitoring, and treatment decisions.
Background: why kidney trajectories matter in HFrEF
Heart failure with reduced ejection fraction (HFrEF) and chronic kidney disease (CKD) commonly coexist. Declining renal function is an established prognostic marker in HF: lower baseline estimated glomerular filtration rate (eGFR) and episodes of worsening kidney function are both associated with higher morbidity and mortality. However, most clinical practice and guideline recommendations focus on cross-sectional eGFR thresholds (for example, when to modify or avoid certain drugs) rather than on longitudinal eGFR behavior. Understanding whether kidney dysfunction precedes clinical HF deterioration could offer a window for earlier intervention.
Study design and populations
The study by Kobayashi et al. (Eur Heart J. 2025) examined individual-patient eGFR trajectories before and after HF-related events using data from two randomized clinical trial datasets (EPHESUS and EMPHASIS-HF) and a real-world HF cohort (BARCELONA).
– EPHESUS and EMPHASIS-HF cohorts (combined n=8,587) provided repeated creatinine/eGFR measures over a median follow-up of about 17 months.
– The BARCELONA registry (n=2,048) supplied longer-term, real-world follow-up (median 47 months).
HF-related events were defined as HF hospitalization or HF death. The investigators modeled linear changes in eGFR (mL/min/1.73 m² per year) in the 12 months before and 12 months after an HF event, and compared trajectories between those with and without events. They also explored clinical correlates such as New York Heart Association (NYHA) class.
Key results
Event rates and cohort context
HF-related events occurred in 14.1% of patients in the combined trial datasets (EPHESUS/EMPHASIS-HF; median follow-up 17.1 months) and in 33.8% of patients in BARCELONA (median follow-up 47.0 months). These rates reflect different follow-up durations and the contrast between trial-selected and real-world populations.
Pre-event eGFR decline
Across both trial and registry cohorts, patients who later experienced an HF-related event had markedly steeper eGFR declines in the year before the event than those who did not:
- EPHESUS/EMPHASIS-HF: average pre-event slope -4.83 mL/min/1.73 m²/year vs -1.18 mL/min/1.73 m²/year in patients without HF events.
- BARCELONA: average pre-event slope -5.77 mL/min/1.73 m²/year vs -1.35 mL/min/1.73 m²/year in patients without HF events.
These differences are clinically meaningful. A decline of ~4–6 mL/min/1.73 m² over a year is substantially greater than typical age-related decline and may signal pathophysiologic processes relevant to HF destabilization.

Fig.Estimated glomerular filtration rate trajectory before and after heart failure events from the EPHESUS and EMPHASIS-HF trials. HF, heart failure; eGFR, estimated glomerular filtration rate; CI, confidence interval. Number in the figure presents the estimated rate of eGFR decline per year
Post-event eGFR course
In the year following an HF hospitalization or HF death (where measurable), eGFR generally continued to fall but at a slower average rate than pre-event: roughly -3.04 to -3.45 mL/min/1.73 m²/year across datasets. This pattern suggests that the processes driving pre-event kidney decline persist after discharge but may be partially attenuated by in-hospital management.

Fig.
Fig. New York Heart Association class trajectory before and after heart failure events from the EPHESUS and EMPHASIS-HF trials. HF, heart failure; NYHA, New York Heart Association; CI, confidence interval. Dotted lines indicate 1 year before and after HF events. Number in the figure presents the estimated rate of estimated glomerular filtration rate decline per year
Symptom burden and kidney slope
Worsening NYHA class paralleled steeper eGFR declines prior to HF events, supporting the clinical link between symptomatic congestion and deterioration in renal function — consistent with cardiorenal interactions driven by elevated venous pressures and reduced renal perfusion.
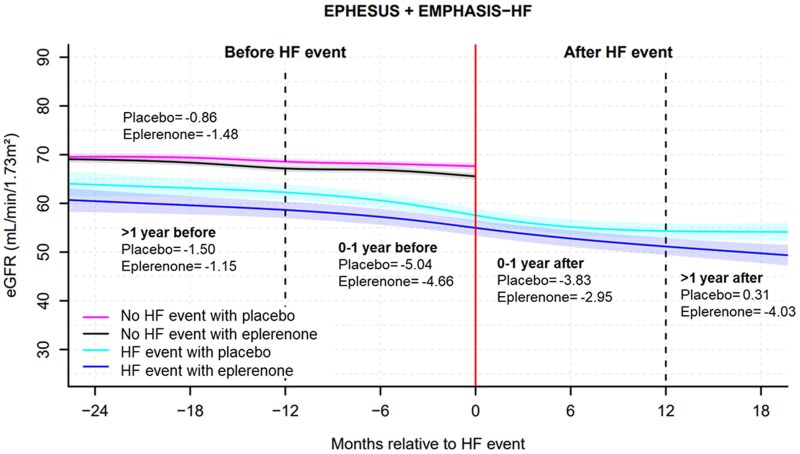
Fig. Estimated glomerular filtration rate trajectory before and after heart failure events between eplerenone and placebo groups from the EPHESUS and EMPHASIS-HF trials. HF, heart failure, Number in the figure presents the estimated rate of estimated glomerular filtration rate decline per year in the placebo/eplerenone groups
Interpretation and mechanistic plausibility
The consistent finding across randomized-trial and real-world cohorts that eGFR declines markedly in the 12 months before HF hospitalization or HF death supports a temporal and likely mechanistic link. Several pathophysiologic explanations are plausible:
- Congestion-driven renal impairment: elevated central venous and renal venous pressures reduce effective filtration and can lead to progressive renal dysfunction before overt decompensation.
- Lower cardiac output and renal hypoperfusion: chronic reduction in forward flow contributes to progressive kidney injury.
- Neurohormonal activation and inflammation: chronic activation of RAAS, sympathetic nervous system, and proinflammatory pathways may drive both cardiac and renal structural and functional decline.
- Treatment and diagnostic factors: changes in diuretic dosing, RAAS inhibitor initiation/withdrawal, nephrotoxins, or intercurrent illnesses may accelerate eGFR decline and precipitate hospitalization.
This study reinforces the concept of the cardiorenal continuum where renal deterioration is not merely a bystander but part of the trajectory toward HF events.
Clinical implications
Several practical implications arise from these findings:
- Monitor eGFR slopes, not only absolute thresholds. Serial measurements that reveal an accelerated negative slope over months may identify patients at higher short-term risk of HF hospitalization.
- Integrate eGFR trajectories into risk alerts and care pathways. Electronic health records could flag patients whose eGFR decline exceeds expected age-related loss or an individualized baseline trajectory, prompting clinician review.
- Assess for congestion when kidney decline is detected. Because congestion appears linked to pre-event renal decline, clinicians should evaluate volume status and consider earlier outpatient diuretic adjustment or review adherence and weight trends.
- Be cautious in interpreting creatinine rises after initiation of RAAS inhibitors or SGLT2 inhibitors. Some drug-related early creatinine increases do not mandate immediate cessation; clinical context and trajectory matter.
- Consider multidisciplinary care. Closer collaboration between heart failure teams and nephrology may be beneficial for patients with rapidly falling eGFR.
Limitations and uncertainties
While compelling, the findings require cautious interpretation:
- Observational trajectory analyses cannot fully establish causation; while eGFR decline precedes HF events, it may reflect shared pathophysiology rather than a causal driver.
- Measurement frequency and timing vary between trial and registry settings, and less frequent outpatient creatinine checks may miss shorter-term fluctuations.
- Acute rises in creatinine related to transient factors (e.g., intercurrent infection, nephrotoxins, dehydration) can confound slopes if not contextualized clinically.
- Generalizability to HFpEF or non-HFrEF populations is unknown; cohorts studied were HFrEF populations.
- Optimal thresholds for an abnormal eGFR slope (that would trigger action) are not established and need prospective validation.
Research and practice gaps
Key next steps include:
- Prospective validation studies that define actionable eGFR slope thresholds, assess sensitivity and specificity for impending HF events, and test intervention strategies triggered by slope alerts.
- Randomized trials of early decongestive or multidisciplinary interventions prompted by eGFR decline to determine whether this strategy prevents HF hospitalization.
- Integration of eGFR slopes with other biomarkers (natriuretic peptides, weight, bioimpedance) and remote monitoring data to build robust predictive models.
Practical takeaways for clinicians
– Review serial eGFR values over months rather than a single laboratory result. A progressive decline of several mL/min/1.73 m²/year should prompt a reassessment of congestion, adherence, medication changes, and comorbid contributors.
– When eGFR falls, consider intensifying clinical review (phone, clinic visit) and objective congestion assessment rather than reflexively stopping guideline-directed HF therapy.
– Use multidisciplinary input early for patients with rapid eGFR decline, and consider nephrology consultation when trajectories are steep or when additive renal insults are suspected.
Conclusion
The study by Kobayashi et al. shows that in HFrEF, clinically meaningful kidney function decline often predates HF-related hospitalization or death by up to a year, and that eGFR continues to fall after such events. Serial eGFR trajectories provide actionable prognostic information that complements existing assessments of symptoms and natriuretic peptides. Incorporating slope-based monitoring into routine care—backed by prospective validation and integrated clinical pathways—could permit earlier identification of patients at risk for decompensation and enable timely, targeted interventions.
Funding and clinicaltrials.gov
Refer to the original publication for detailed funding sources and trial registrations for EPHESUS and EMPHASIS-HF.
References
1. Kobayashi M, Bayes-Genis A, Duarte K, McMurray JJV, Ferreira JP, Pocock SJ, Van Veldhuisen DJ, Lupón J, Pitt B, Zannad F, Girerd N. Kidney function trajectories before and after hospitalization for heart failure with reduced ejection fraction. Eur Heart J. 2025 Nov 14;46(43):4583-4593. doi: 10.1093/eurheartj/ehaf457 IF: 35.6 Q1 .
2. McDonagh TA, Metra M, Adamo M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42(36):3599–3726.
3. McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N Engl J Med. 2019;381:1995-2008.
4. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Suppl. 2013;3(1):1–150.