Highlights of the 10-Year FIMPACT Follow-Up
The Finnish Shoulder Impingement Arthroscopy Controlled Trial (FIMPACT) provides landmark data on the long-term efficacy of one of the most common orthopedic procedures. The key highlights include:
- At the 10-year mark, arthroscopic subacromial decompression (ASD) showed no clinically significant benefit over placebo surgery (diagnostic arthroscopy) for pain at rest or during activity.
- Surgical decompression was not superior to a structured exercise therapy program, reinforcing the value of conservative management.
- The findings demonstrate that the initial perceived benefits of ASD are likely attributable to the placebo effect, natural history of the condition, or regression to the mean rather than the mechanical removal of the acromion.
- Safety profiles and secondary functional outcomes were comparable across all three groups, suggesting no long-term ‘hidden’ benefit to surgical intervention.
Introduction: The Rise and Fall of the Impingement Theory
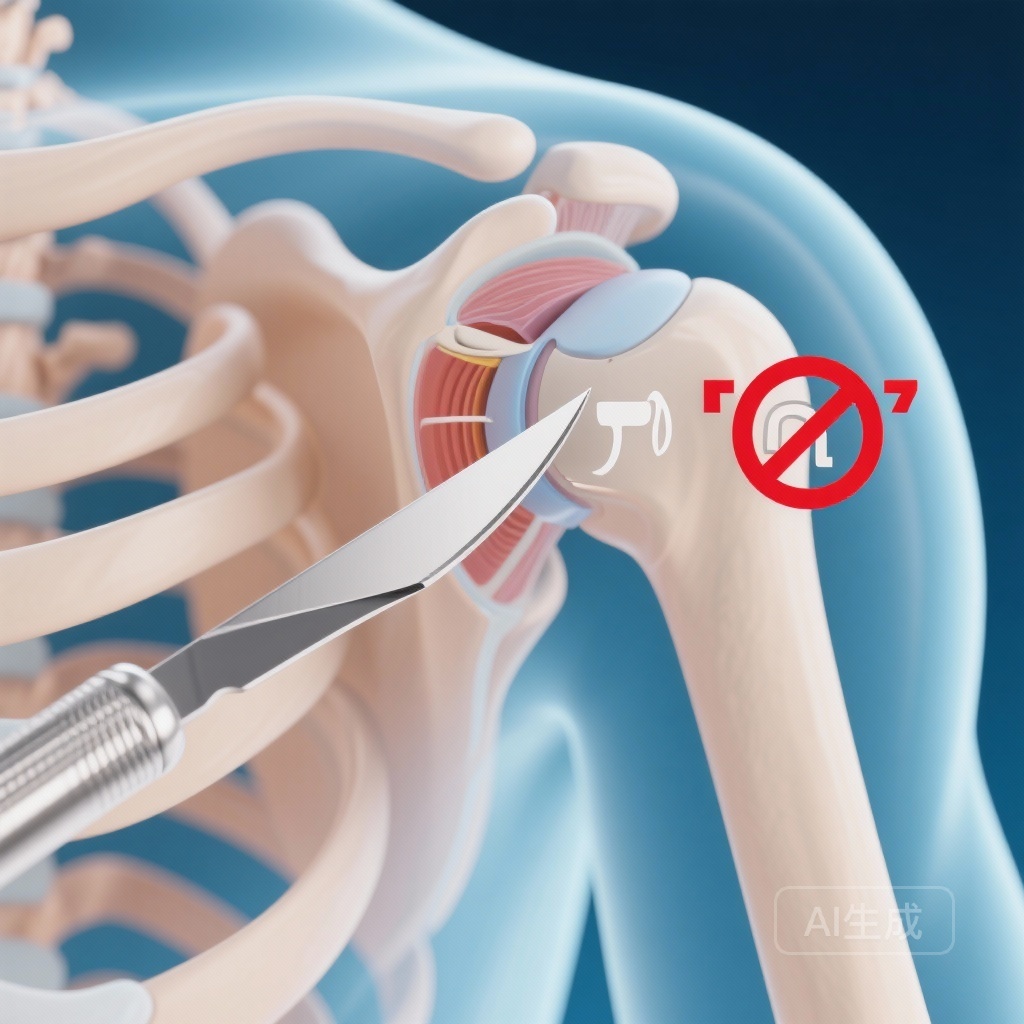
For decades, subacromial pain syndrome (SAPS)—traditionally known as shoulder impingement—has been managed under the biomechanical premise that the acromion process mechanically abrades the rotator cuff tendons. This theory, popularized in the 1970s by Charles Neer, led to the widespread adoption of arthroscopic subacromial decompression (ASD). The procedure involves shaving away part of the bone to “increase the space” for the tendons.
However, despite its ubiquity, the scientific foundation for ASD has faced increasing scrutiny. Critics argue that acromial shape is often a normal anatomical variation or a secondary result of rotator cuff pathology rather than the primary cause of pain. As evidence-based medicine has evolved, several short-term trials suggested that decompression might not be superior to non-operative care. The FIMPACT trial was designed to provide the definitive answer by incorporating a placebo (sham) surgery arm and extending follow-up to a full decade.
Study Design: A Rigorous Approach to Surgical Efficacy
The FIMPACT trial is a multicenter, randomized, placebo-surgery controlled study conducted across three public hospitals in Finland. The study design represents the gold standard for evaluating surgical interventions, where the psychological and physiological effects of the incision and anesthesia are controlled for.
Participants and Setting
The trial enrolled 210 adults between the ages of 35 and 65 who had experienced symptoms of subacromial pain for at least three months despite conservative treatment. Recruitment began in 2005, with the 10-year follow-up concluding in late 2023. This longitudinal perspective is critical for chronic musculoskeletal conditions where symptoms may fluctuate over years.
Interventional Arms
Participants were randomized into three distinct groups:
- Arthroscopic Subacromial Decompression (ASD): Standard surgical removal of the subacromial bursa and ligament, along with bone resection of the anterior acromion.
- Placebo Surgery (Sham): A diagnostic arthroscopy where the surgeon performed a systematic examination of the joint but did not perform any decompression or tissue removal. Patients and outcome assessors remained blinded to this allocation.
- Exercise Therapy: A pragmatic comparator involving a supervised, structured exercise program focusing on rotator cuff strengthening and scapular stabilization.
Key Findings: A Decade of Longitudinal Data
The primary objective was to assess shoulder pain at rest and during activity using a Visual Analogue Scale (VAS) from 0 to 100. A difference of 15 points was predefined as the minimally important difference (MID) for clinical relevance.
Primary Outcomes: Pain at Rest and Activity
In the primary intention-to-treat analysis comparing ASD to placebo surgery, the results were striking in their lack of divergence. At the 10-year follow-up, 168 participants (87% of the original cohort) were assessed. The mean difference between the ASD and placebo groups for pain at rest was a mere -1.5 points (95% CI -8.6 to 5.6). For pain during arm activity, the difference was -3.2 points (95% CI -13.0 to 6.5). Both values fall well below the threshold of clinical significance.
ASD versus Exercise Therapy
The pragmatic comparison between surgery and exercise therapy yielded similar results. The mean difference for pain at rest was -4.0 points, and for pain during activity, it was -9.4 points. While the numerical trend slightly favored surgery over exercise in the activity category, the confidence intervals crossed zero, and the magnitude did not reach the 15-point MID required to justify the risks and costs of an invasive procedure.
Secondary Outcomes and Safety
Beyond pain scores, the trial examined secondary outcomes including functional scores (such as the Constant-Murley score) and the incidence of adverse events. No significant between-group differences were found. Furthermore, there were no long-term safety concerns specific to any group, but the lack of superior efficacy in the surgical group renders the inherent surgical risks (infection, anesthesia complications) unnecessary for the average patient with SAPS.
Discussion: Reinterpreting Subacromial Pain
The FIMPACT 10-year data provides a robust rebuttal to the mechanical impingement model. If removing the “impinging” bone does not lead to better outcomes than a sham procedure over ten years, the mechanical model must be flawed.
The Power of the Placebo Effect in Surgery
The fact that patients in the placebo surgery group improved significantly from baseline suggests that the “surgical experience” itself—including the ritual of the operating room, the anesthesia, and the postoperative care—has a profound psychological impact. Additionally, the natural history of subacromial pain often involves gradual resolution or adaptation over time, which may be falsely attributed to the surgical intervention by both patients and clinicians.
Conservative Management as the Gold Standard
The exercise therapy arm performed remarkably well, matching surgical outcomes without the need for invasive measures. This suggests that the physiological benefits of exercise—improving tendon load capacity, neuromuscular control, and blood flow—are the primary drivers of recovery in SAPS.
Expert Commentary: Shifting the Clinical Paradigm
These findings align with other major trials, such as the CSAW trial in the UK, but provide much-needed long-term validation. Many clinicians have been hesitant to abandon ASD, fearing that symptoms might return years later if the bone is not removed. FIMPACT effectively silences those concerns.
However, an evidence-practice gap remains. ASD continues to be performed at high rates globally. Experts suggest that health policy must shift toward “de-implementation” of low-value surgical procedures. Guidelines should clearly state that for patients with subacromial pain without full-thickness rotator cuff tears, surgery should not be offered as a routine treatment, even if conservative care fails, because the surgery itself appears to be a placebo.
Conclusion: The End of an Era for ASD?
The 10-year follow-up of the FIMPACT trial is a definitive piece of evidence in modern orthopedics. It confirms that arthroscopic subacromial decompression provides no clinical advantage over placebo surgery or exercise for patients with subacromial pain syndrome. Clinicians should prioritize high-quality exercise programs and patient education, reassuring patients that “bone-on-bone” impingement is a largely discredited concept in this context. The future of shoulder care lies in movement and rehabilitation, not the scalpel.
Funding and Trial Registration
The FIMPACT trial was supported by the Academy of Finland, the Sigrid Jusélius Foundation, and various Finnish institutional research grants. The trial is registered at ClinicalTrials.gov (NCT00428870).
References
1. Kanto K, Bäck M, Ibounig T, et al. Arthroscopic subacromial decompression versus placebo surgery for subacromial pain syndrome: 10 year follow-up of the FIMPACT randomised, placebo surgery controlled trial. BMJ. 2025;391:e086201.
2. Beard DJ, Rees JL, Cook JA, et al. Arthroscopic subacromial decompression for subacromial shoulder pain (CSAW): a multicentre, pragmatic, parallel group, placebo-controlled, randomised surgical trial. Lancet. 2018;391(10118):329-338.
3. Paavola M, Malmivaara A, Taimela S, et al. Subacromial decompression versus diagnostic arthroscopy for shoulder impingement: randomised, placebo surgery controlled clinical trial. BMJ. 2018;362:k2860.