Highlights
- Functional connectivity from resting-state fMRI robustly predicts both cross-sectional tau burden and longitudinal tau accumulation in atypical Alzheimer’s disease (AD) variants.
- Distinct tau-PET epicentres correspond to clinical phenotypes—visual network for posterior cortical atrophy (PCA), language network for logopenic variant primary progressive aphasia (lvPPA), and other relevant networks for behavioural and corticobasal syndromes.
- Post-mortem tau pathology analysis corroborates in vivo PET findings, reinforcing connectivity-guided tau propagation as a universal mechanism across atypical AD presentations.
- These insights support the development of personalized biomarkers and participant-specific endpoints for clinical trials targeting tauopathies in AD.
Background
Alzheimer’s disease (AD) is characterized by progressive accumulation of amyloid-β plaques and tau neurofibrillary tangles, which correlate more closely with neurodegeneration and clinical symptoms. Typical AD predominantly affects memory through temporal lobe involvement, with a well-characterized stereotypical pattern of tau spread. However, atypical AD variants, including posterior cortical atrophy (PCA), logopenic variant primary progressive aphasia (lvPPA), behavioural variant AD (bvAD), and corticobasal syndrome AD (CBS-AD), present with divergent clinical phenotypes and spatial tau deposition patterns. Understanding the underlying mechanisms guiding tau propagation in these heterogeneous forms is critical for advancing diagnosis and therapy.
Previous studies have suggested that tau spreads transneuronally along functionally connected networks, mainly described in typical AD. Yet, whether this connectivity-based model generalizes to atypical AD forms remained unclear due to their distinct tau topographies and clinical manifestations.
Key Content
Study Design and Population
A landmark multicentre study by de Bruin et al. incorporated data from 320 individuals clinically diagnosed with atypical AD subtypes: 139 with PCA, 103 with lvPPA, 35 with bvAD, and 43 with CBS-AD, collected across 14 international sites. Longitudinal tau-PET imaging was available for 78 participants, strengthening temporal inferences of tau propagation. An independent post-mortem cohort comprising 93 atypical AD patients provided pathological validation.
Methodological Advances
Multiple tau-PET tracers were harmonized using Gaussian mixture modelling to convert standardized uptake value ratios (SUVRs) into tau positivity probabilities ranging from 0% to 100%, enabling standardized assessment across heterogeneous imaging protocols. Resting-state functional MRI (rs-fMRI) connectivity patterns from healthy elderly controls in the Alzheimer’s Disease Neuroimaging Initiative (ADNI) served as a normative connectivity scaffold.
Linear regression analyses evaluated the covariance of tau load and accumulation in brain regions with respect to their functional connectivity strength. Importantly, tau-PET epicentres (top 5% regions by baseline tau load) and accumulation epicentres (top 5% regions by tau accumulation rate) were identified in each clinical variant to map propagation trajectories.
Figure 1. Tau-PET epicentres and positivity across AD variants.
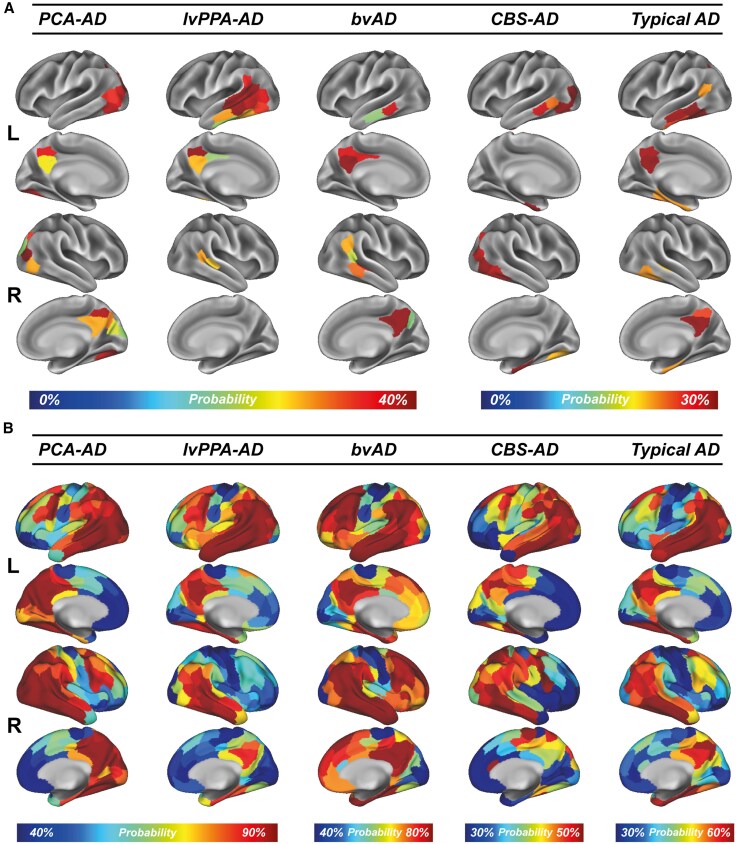
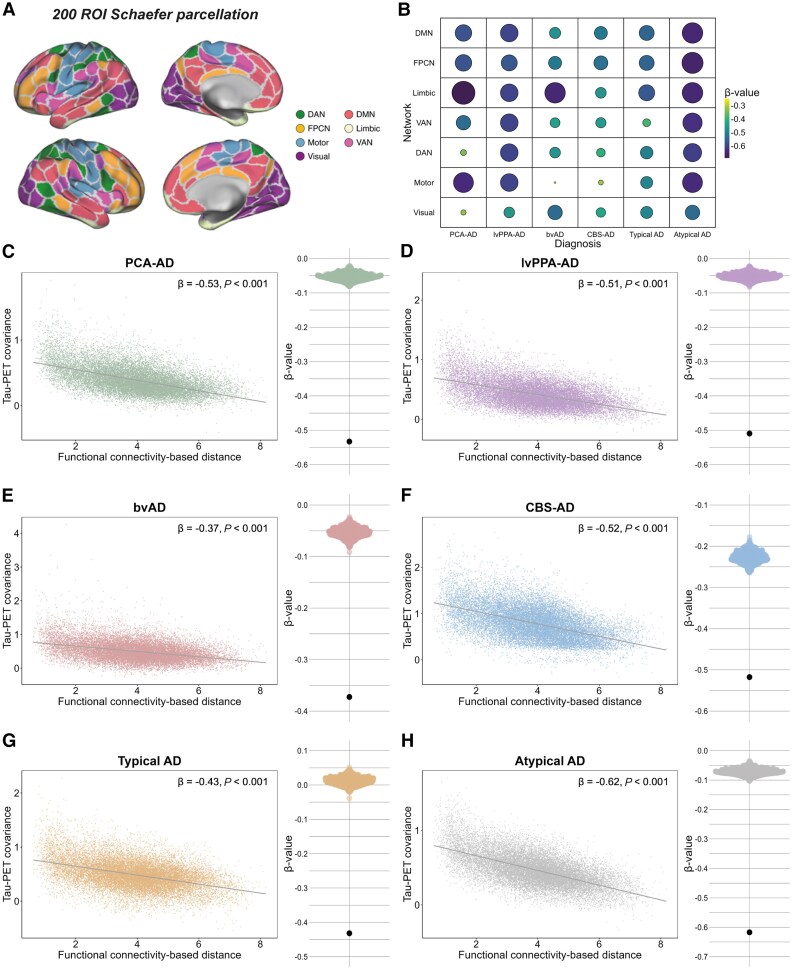
Figure 2. Association between functional connectivity and covariance in tau-PET across variants of AD.
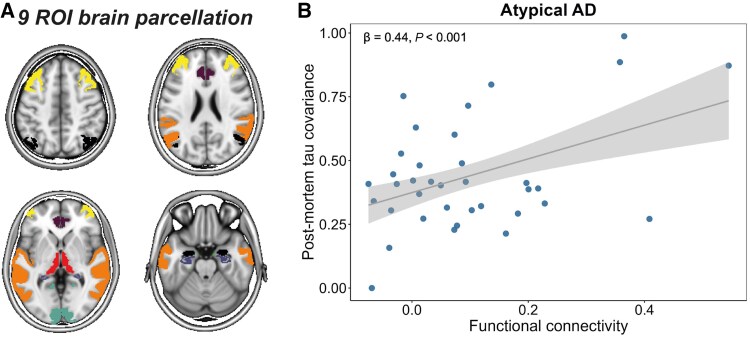
Figure 3. Association between functional connectivity and covariance in post-mortem tau pathology in atypical AD.
Tau Epicentres and Clinical Phenotypes
– PCA-AD exhibited tau epicentres concentrated in the visual network, explaining prominent visuospatial deficits.
– lvPPA-AD showed left-hemispheric temporal predominance aligning with the language network, consistent with aphasic features.
– bvAD and CBS-AD patterns highlighted frontotemporal and motor-related regions, respectively.
These regional specializations underpin the clinical heterogeneity and validate the notion that tau epicentres correspond to variant-specific vulnerabilities.
Connectivity Predicts Tau Spread
Analyses revealed that brain regions with stronger functional connectivity to tau epicentres had greater concurrent tau accumulation both cross-sectionally and over time, across all atypical AD variants. This relationship held true when calibrated against post-mortem tau burden, confirming in vivo imaging results.
Functionally proximal regions exhibited elevated tau positivity probability and higher accumulation rates, whereas functionally distant regions remained relatively spared. These findings support a model of trans-synaptic tau propagation following the brain’s intrinsic connectivity architecture.
Implications for Personalized Medicine and Trials
By pinpointing tau propagation pathways specific to each clinical phenotype, this connectivity-based framework offers a robust mechanistic basis for individualizing diagnosis and monitoring progression. Tau-PET epicentre and connectivity profiles can inform participant selection and tailored endpoints in therapeutic trials aimed at halting tau spread.
Expert Commentary
This study substantially advances the field by generalizing the connectivity-driven tau propagation hypothesis beyond typical AD to multiple atypical variants. The use of harmonized tau-PET metrics and the integration of longitudinal and post-mortem data enhance confidence in these findings.
From a mechanistic standpoint, the preferential tau spread along functional networks provides a plausible neuropathological substrate for clinical manifestations, reflecting selective network vulnerability. It also underscores the importance of network-based frameworks in future biomarker development and therapeutic targeting.
Limitations include the observational nature precluding causal inference and reliance on normative connectivity data rather than patient-specific connectomes, which may differ due to neurodegeneration. Additionally, distinguishing primary tau propagation from secondary neurodegenerative processes remains challenging.
Nonetheless, the results advocate incorporating functional connectivity assessments into clinical evaluation and trial designs, moving towards participant-specific tau progression models.
Conclusion
The comprehensive multicentre investigation demonstrates that functional connectivity universally predicts the spatial and temporal trajectory of tau pathology in atypical AD variants. This connectivity scaffold governs tau spread from clinical phenotype-defining epicentres to functionally connected regions, aligning closely with clinical presentations.
This paradigm shift refines our understanding of tau pathology propagation, facilitating precise stratification and personalized interventions in AD. Future studies integrating patient-derived connectivity measures and multimodal biomarkers will be critical to translate these insights into clinical practice and effective disease-modifying therapies.
References
- de Bruin H, Groot C, Barthel H, et al. Connectivity as a universal predictor of tau progression in atypical Alzheimer’s disease. Brain. 2025;148(11):3893-3912. doi:10.1093/brain/awaf279 IF: 11.7 Q1 . PMID: 40810361 IF: 11.7 Q1 ; PMCID: PMC12588720 IF: 11.7 Q1 .
- Brier MR, Gordon B, Friedrichsen K, et al. Tau pathology and functional connectivity correspond to clinical symptoms in Alzheimer disease. Neurology. 2016;87(19):1967-1974. doi:10.1212/WNL.0000000000003269 IF: 8.5 Q1 .
- Jones DT, Graff-Radford J, Lowe VJ, et al. Tau, amyloid, and cascading network failure across the Alzheimer’s disease spectrum. Cortex. 2017;97:143-159. doi:10.1016/j.cortex.2017.08.022 IF: 3.3 Q1 .
- Whitwell JL, Jack CR Jr, Boeve BF, et al. Imaging correlates of posterior cortical atrophy. Neurobiol Aging. 2007;28(7):1051-1061. doi:10.1016/j.neurobiolaging.2006.06.018 IF: 3.5 Q2 .