Introduction: The Diagnostic Dilemma of Chronic Bronchitis
Chronic bronchitis (CB) has traditionally been defined by clinical symptoms—specifically, a productive cough for at least three months in two consecutive years. While this definition has served clinicians for decades, it is inherently subjective and often fails to capture the underlying biological reality of muco-obstructive lung disease. Recent advances in respiratory medicine have focused on identifying objective biomarkers that can quantify airway mucus pathology. Previous research established that total sputum mucin concentration is a reliable marker for CB. However, a new study published in NEJM Evidence introduces a more sophisticated metric: the Mucin Quantitative Score (MUCQ). This score integrates both the quantity and the specific molecular composition of mucus, providing a superior diagnostic tool for clinicians and researchers alike.
Highlights of the MUCQ Metric
The study’s findings underscore a pivotal shift toward precision medicine in pulmonary care. Key takeaways include:
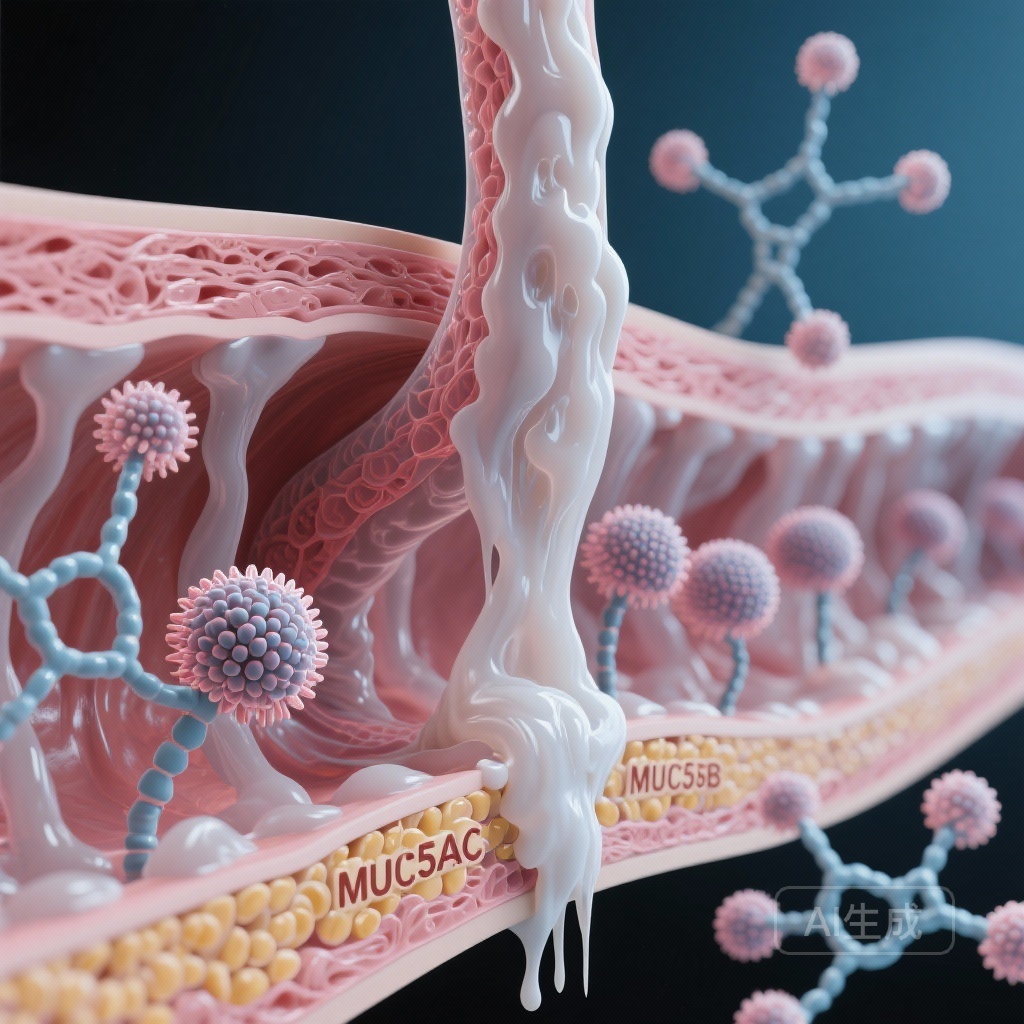
- The MUCQ score incorporates the ratio of MUC5AC to MUC5B, accounting for qualitative changes in mucus that drive airway obstruction.
- In the SPIROMICS cohort, MUCQ significantly improved the reclassification of patients, accurately identifying chronic bronchitis in individuals who were previously misclassified by total mucin concentration alone.
- The metric demonstrates a strong correlation with objective pathological indices, including airway obstruction and lung function decline.
- MUCQ offers a standardized tool for monitoring therapeutic responses in clinical trials targeting mucus hypersecretion.
Background: The Pathophysiology of Mucin
To understand the significance of MUCQ, one must understand the roles of the two primary gel-forming mucins in the human lung: MUC5B and MUC5AC. MUC5B is the dominant mucin in healthy airways, essential for normal mucociliary clearance and innate defense. In contrast, MUC5AC is highly inducible and typically upregulated in response to inflammatory stimuli, such as cigarette smoke, allergens, or viral infections. In chronic obstructive pulmonary disease (COPD) and CB, the overproduction of MUC5AC relative to MUC5B results in mucus that is more viscous, more adherent to the airway wall, and more difficult to clear. This imbalance is a primary driver of airway plugging and subsequent lung function decline. The MUCQ score was developed specifically to capture this biological shift.
Study Design and Methodology
The researchers utilized data from the SubPopulations and InteRmediate Outcome Measures in COPD Study (SPIROMICS) cohort. Participants were initially classified as having CB or not based on standardized clinical questionnaires. Sputum samples were collected and analyzed for total mucin concentration, as well as individual concentrations of MUC5AC and MUC5B.
The MUCQ Equation
The novel MUCQ score was calculated using the following formula: [Total mucin] × ([MUC5AC] ÷ [MUC5B]) ÷ 100 μg/ml. This unitless, weighted concentration score allows the composition of the mucus to modulate the impact of the total concentration. The study aimed to determine if MUCQ could better distinguish CB patients than the previous gold-standard threshold for total mucin (2306 μg/ml).
Primary Outcomes
The primary outcome was the net reclassification of patients. Participants were first categorized using the total mucin threshold and then re-evaluated using a MUCQ threshold of 4.30. The researchers applied z statistics and calculated p-values to assess the clinical significance of this reclassification.
Key Results: Superior Diagnostic Accuracy
The results from the 164 patients in the SPIROMICS cohort with clinically defined CB were striking. The use of the MUCQ score led to a significant redistribution of patient classifications. Specifically, the MUCQ metric up-classified 18 patients who were current smokers to a diagnosis of CB—patients who would have been missed if only total mucin concentration was measured. Conversely, MUCQ down-classified 5 current smokers and 3 never-smoker controls who had high total mucin but a low MUC5AC/MUC5B ratio, suggesting their elevated mucin was not representative of the pathological CB state.
The statistical analysis yielded a P value of 0.001, confirming that MUCQ is a statistically superior metric for identifying the CB phenotype. Beyond simple classification, the MUCQ score showed a linear correlation with other clinical indices of chronic airway disease. Higher MUCQ scores were associated with increased airway obstruction, as measured by FEV1/FVC ratios, and increased symptom burden as reported on the COPD Assessment Test (CAT).
Expert Commentary: Mechanistic Insights
The biological plausibility of the MUCQ score lies in its focus on the MUC5AC:MUC5B ratio. In healthy lungs, the airway surface liquid is a finely tuned system. When MUC5AC increases, the physical properties of the mucus change. MUC5AC is known to be more ‘tethered’ to the goblet cells that produce it, leading to the formation of mucus strands that are resistant to clearance. By weighting the total mucin concentration by this ratio, MUCQ effectively identifies ‘low-quality’ mucus that is more likely to cause clinical symptoms and physiological impairment. Experts suggest that this metric could serve as a surrogate endpoint in trials for new mucolytic therapies or anti-inflammatory agents designed to normalize mucin production.
Conclusion and Future Directions
The introduction of the MUCQ score represents a significant advancement in the objective assessment of chronic bronchitis. By moving away from subjective questionnaires and simplistic concentration measurements, clinicians can now leverage a metric that reflects the true molecular pathology of the airways. While the SPIROMICS data provides a strong foundation, prospective trials are now needed to determine how MUCQ can be integrated into routine clinical practice for the long-term management of COPD and other muco-obstructive conditions like bronchiectasis and cystic fibrosis. As we enter an era of targeted biological therapies, having a precise, composition-based biomarker like MUCQ will be essential for identifying the right patients for the right treatments.
Funding and ClinicalTrials.gov
This research was funded by the National Institutes of Health (NIH), the National Heart, Lung, and Blood Institute (NHLBI), and the National Institute of Environmental Health Sciences (NIEHS). The SPIROMICS study is registered at ClinicalTrials.gov (NCT01969331).